Page 107 - Hydrogeology Principles and Practice
P. 107
HYDC03 12/5/05 5:36 PM Page 90
90 Chapter Three
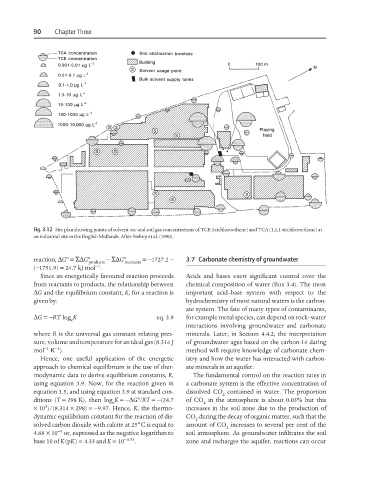
Fig. 3.12 Site plan showing points of solvent use and soil gas concentrations of TCE (trichloroethene) and TCA (1,1,1-trichloroethane) at
an industrial site in the English Midlands. After Bishop et al. (1990).
o
reaction, ∆G =Σ∆G o −Σ∆G o =−1727.2 − 3.7 Carbonate chemistry of groundwater
products reactants
−1
(−1751.9) = 24.7 kJ mol .
Since an energetically favoured reaction proceeds Acids and bases exert significant control over the
from reactants to products, the relationship between chemical composition of water (Box 3.4). The most
∆G and the equilibrium constant, K, for a reaction is important acid–base system with respect to the
given by: hydrochemistry of most natural waters is the carbon-
ate system. The fate of many types of contaminants,
∆G =−RT log K eq. 3.9 for example metal species, can depend on rock–water
e
interactions involving groundwater and carbonate
where R is the universal gas constant relating pres- minerals. Later, in Section 4.4.2, the interpretation
sure, volume and temperature for an ideal gas (8.314 J of groundwater ages based on the carbon-14 dating
−1
−1
mol K ). method will require knowledge of carbonate chem-
Hence, one useful application of the energetic istry and how the water has interacted with carbon-
approach to chemical equilibrium is the use of ther- ate minerals in an aquifer.
modynamic data to derive equilibrium constants, K, The fundamental control on the reaction rates in
using equation 3.9. Now, for the reaction given in a carbonate system is the effective concentration of
equation 3.5, and using equation 3.9 at standard con- dissolved CO contained in water. The proportion
2
o
ditions (T = 298 K), then log K =−∆G /RT =−(24.7 of CO in the atmosphere is about 0.03% but this
e 2
3
× 10 )/(8.314 × 298) =−9.97. Hence, K, the thermo- increases in the soil zone due to the production of
dynamic equilibrium constant for the reaction of dis- CO during the decay of organic matter, such that the
2
solved carbon dioxide with calcite at 25°C is equal to amount of CO increases to several per cent of the
2
−5
4.68 × 10 or, expressed as the negative logarithm to soil atmosphere. As groundwater infiltrates the soil
base 10 of K (pK) = 4.33 and K = 10 −4.33 . zone and recharges the aquifer, reactions can occur