Page 108 - Hydrogeology Principles and Practice
P. 108
HYDC03 12/5/05 5:36 PM Page 91
Chemical hydrogeology 91
BO X
Active concentration
3.3
The active concentration or activity of an ion is an important con- I S c z 2 eq. 2
=
1
sideration in concentrated and complex solutions such as seawater, 2 i ii
but also for groundwaters and surface waters that contain dissolved
−1
where c is the concentration of ion, i, in mol L , z , is the charge
ions from many sources. Ions in a concentrated solution are suf- i i
of ion, i, and S represents the sum of all ions in the solution. As a
ficiently close to one another for electrostatic interactions to occur.
measure of the concentration of a complex electrolyte solution,
These interactions reduce the effective concentration of ions avail-
ionic strength is better than a simple sum of molar concentrations
able to participate in chemical reactions and, if two salts share a
since it accounts for the effect of the charge of multivalent ions.
common ion, they mutually reduce each other’s solubility and
Freshwaters typically have ionic strengths between 10 −3 and 10 −4
exhibit the common ion effect. In order to predict accurately chem-
−1
mol L , whereas seawater has a fairly constant ionic strength of
ical reactions in a concentrated solution, it is necessary to account
−1
0.7 mol L .
for the reduction in concentration as follows:
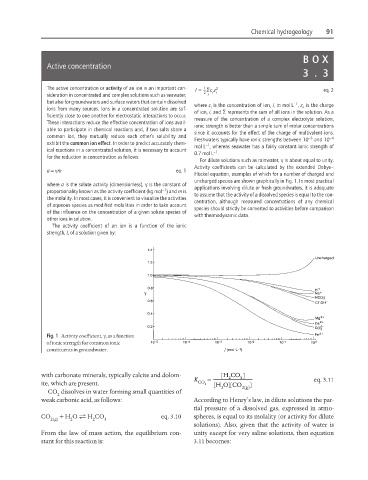
For dilute solutions such as rainwater, g is about equal to unity.
Activity coefficients can be calculated by the extended Debye–
a = gm eq. 1
Hückel equation, examples of which for a number of charged and
uncharged species are shown graphically in Fig. 1. In most practical
where a is the solute activity (dimensionless), g is the constant of applications involving dilute or fresh groundwaters, it is adequate
−1
proportionality known as the activity coefficient (kg mol ) and m is
to assume that the activity of a dissolved species is equal to the con-
the molality. In most cases, it is convenient to visualize the activities
centration, although measured concentrations of any chemical
of aqueous species as modified molalities in order to take account
species should strictly be converted to activities before comparison
of the influence on the concentration of a given solute species of
with thermodynamic data.
other ions in solution.
The activity coefficient of an ion is a function of the ionic
strength, I, of a solution given by:
Fig. 1 Activity coefficient, γ, as a function
of ionic strength for common ionic
constituents in groundwater.
with carbonate minerals, typically calcite and dolom- [ HCO ]
2
3
K = eq. 3.11
ite, which are present. CO 2 [ HO CO ]
][
g
2 2()
CO dissolves in water forming small quantities of
2
weak carbonic acid, as follows: According to Henry’s law, in dilute solutions the par-
tial pressure of a dissolved gas, expressed in atmo-
CO + H O j H CO eq. 3.10 spheres, is equal to its molality (or activity for dilute
2(g) 2 2 3
solutions). Also, given that the activity of water is
From the law of mass action, the equilibrium con- unity except for very saline solutions, then equation
stant for this reaction is: 3.11 becomes: