Page 227 - Industrial Wastewater Treatment, Recycling and Reuse
P. 227
Advanced Treatment Technology and Strategy 201
O .. 2H + −
2
4e
2H O
2
2e − O 2 H O 2
2
−
e 2+
Fe
OH • +
4H +O 2
Fe 3+
Cathode Anode
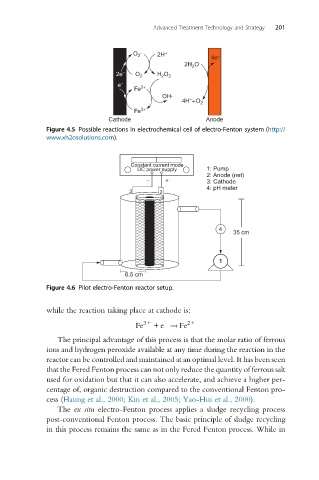
Figure 4.5 Possible reactions in electrochemical cell of electro-Fenton system (http://
www.xh2osolutions.com).
Constant current mode
DC power supply 1: Pump
2: Anode (net)
− + 3: Cathode
4: pH meter
3 2
4
35 cm
1
6.5 cm
Figure 4.6 Pilot electro-Fenton reactor setup.
while the reaction taking place at cathode is:
3+ 2+
Fe +e ! Fe
The principal advantage of this process is that the molar ratio of ferrous
ions and hydrogen peroxide available at any time during the reaction in the
reactor can be controlled and maintained at an optimal level. It has been seen
that the Fered Fenton process can not only reduce the quantity of ferrous salt
used for oxidation but that it can also accelerate, and achieve a higher per-
centage of, organic destruction compared to the conventional Fenton pro-
cess (Haung et al., 2000; Kin et al., 2005; Yao-Hui et al., 2000).
The ex situ electro-Fenton process applies a sludge recycling process
post-conventional Fenton process. The basic principle of sludge recycling
in this process remains the same as in the Fered Fenton process. While in