Page 228 - Inorganic Mass Spectrometry - Fundamentals and Applications
P. 228
Seconda~ Ion Mass Spectrometry 21 3
lo5 I
l
l l l I
0 300 600 900 1200 1500
Depth, nm
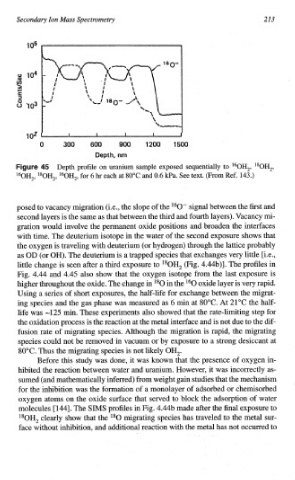
Depth profile on uranium sample exposed sequentially to "OH,, 180H,,
%H,, "*OH,, 160H,, for 6 hr each at 80°C and 0.6 @a. See text. (From Ref. 143.)
posed to vacancy migration (i,e., the slope of the *60" signal between the first and
second layers is the same as that between the third and fourth layers). Vacancy mi-
gration would involve the permanent oxide positions and broaden the interfaces
with time. The deuterium isotope in the water of the second exposure shows that
the oxygen is traveling with deuterium (or hydrogen) through the lattice probably
as OD (or OH). The deuterium is a trapped species that exchanges very little [i.e.,
little change is seen after a third exposure to ISOH, (Fig. 4.44b)I. The profiles in
Fig. 4.44 and 4.45 also show that the oxygen isotope from the last exposure is
higher t~oughout the oxide. The change in l80 in the l60 oxide layer is very rapid.
Using a series of short exposures, the half-life for exchange between the migrat-
ing species and the gas phase was measured as 6 rnin at 80°C. At 21°C the half-
life was -125 rnin. These experiments also showed that the rate-limiting step for
is
the oxidation process is the reaction at the metal interface and not due to the dif-
fusion rate of rnigrating species, Although the migration is rapid, the migrating
species could not be removed in vacuum or by exposure to a strong desiccant at
80°C. Thus the migrating species is not likely OH,.
Before this study was done, it was known that the presence of oxygen in-
it
hibited the reaction between water and uranium. However, was incorrectly as-
sumed (and mathematically inferred) from weight gain studies that the mechanism
for the i~ibition was the formation of a monolayer of adsorbed or chemisorbed
oxygen atoms on the oxide surface that served to block the adsorption of water
molecules [144]. The SIMS profiles in Fig. 4.44b made after the final exposure to
l80H, clearly show that the l80 migrating species has traveled to the metal sur-
face without inhibition, and additional reaction with the metal has not occurred to