Page 163 - Instrumentation Reference Book 3E
P. 163
Absolute gauges 147
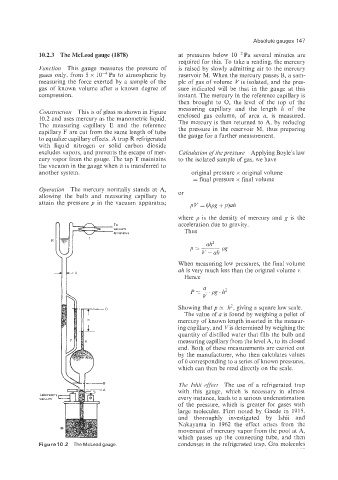
10.2.3 The McLeod gauge (1878) at pressures below 10-2Pa several minutes are
required for this. To take a reading. the mercury
Function This gauge measures the pressure of is raised by slowly admitting air to the mercury
gases only, from 5 x 10-4Pa to atmospheric by reservoir M. When the mercury passes B, a sam-
measuring the force exerted by a sample of the ple of gas of volume Vis isolated, and the pres-
gas of known volume after a known degree of sure indicated will be that in the gauge at this
compression. instant. The mercury in the reference capillary is
then brought to 0, the level of the top of the
Construction This is of glass as shown in Figure measuring capillary and the length h of the
10.2 and uses mercury as the manometric liquid. enclosed gas column, of area a, is measured.
The measuring capillary E and the reference The mercury is then returned to A, by reducing
capillary F are cut from the same length of tube the pressure in the reservoir M, thus preparing
the gauge for a further measurement.
to equalize capillary effects. A trap R refrigerated
with liquid nitrogen or solid carbon dioxide
excludes vapors, and prevents the escape of mer- Calculation of the pressure Applying Boyle’s Iaw
cury vapor from the gauge. The tap T maintains to the isolated sample of gas; we have
the vacuuim in the gauge when it 1s transferred to
another system. original pressure x original volume
= final pressure x final volume
Opevation The mercury normally stands at A,
allowing the bulb and measuring capillary to or
attain the pressure p in the vacuum apparatus; p V = (Izpg + p)ah
where p is the density of mercury and g is the
TO acceleration due to gravity.
vacuum Thus
appararur
R
ah2
p=- V - ah Pg
When measuring low pressures, the final volume
ah is very much less than thle original volume 11.
Hence
a
P = -. pg . h2
V
Showing that p x h2, giving a square law scale.
The value of a is found by weighing a pellet of
mercury of known length inserted in the measur-
ing capillary, and Vis determined by weighing the
quantity of distilled water that fills the bulb and
measuring capillary from the level A. to its closed
end. Both of these measurements are carried out
by the manufacturer, who then calculates values
of h corresponding to a series of known pressures.
which can then be read directly on the scale.
The Islzii effect The use of a refrigerated trap
.I
with this gauge, which is necessary in almost
Laboratory
YaCUlim 1 every instance, leads to a serious underestimation
of the pressure, which is greater for gases with
large molecules. First noted by Gaede in 1915,
and thoroughly investigated by Ishii and
Nakayama in 1962 the effect arises from the
movement of mercury vapor from the pool at A,
which passes up the connecting tube, and then
FigurelO.2 The McLeodgauge. condenses in the refrigerated trap. Gas molecules