Page 324 - Instrumentation Reference Book 3E
P. 324
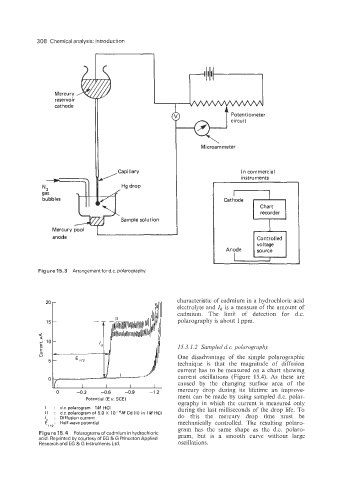
308 Chemical analysis: introduction
cathode
c Potentiometer
circuit
M icroam meter
II,, instruments
In commercial
Capillary
N2 n Hg drop
gas
bubbles Cathode
Chart
recorder
Sample solution
Mercury pool I
I
anode Controlled
voltage
Figure 15.3 Arrangementf0rd.c. polarography.
characteristic of cadmium in a hydrochloric acid
electrolyte and Id is a measure of the amount of
cadmium. The limit of detection for d.c.
polarography is about 1 ppm.
15.3.1.2 Sainpled d.c. polnrogrrrplqv
One disadvantage of the simple polarographic
technique is that the magnitude of diffusion
current has to be measured on a chart showing
current oscillations (Figure 15.4). As these are
caused by the changing surface area of the
0 -0.3 -0.6 -0.9 -1.2 mercury drop during its lifetime an improve-
Potential (E v. SCE) ment can be made by using sampled d.c. polar-
ography in which the current is measured only
I : d.c polarogram tM HCI during the last milliseconds of the drop life. To
II : d c polarogram of 5.0 X lO-'M Cd (ii) in 1M HCI
Id : Diffusion current do this the mercury drop time must be
E,,2: Half-wave potential mechanically controlled. The resulting polaro-
gram has the same shape as the d.c. polaro-
Figure 15.4 Polarogramsof cadmium in hydrochloric
acid. Reprinted by courtesy of EG & G Princeton Applied gram, but is a smooth curve without large
Research and EG & G Instruments Ltd. oscillations.