Page 370 - Instrumentation Reference Book 3E
P. 370
Electrical conductivity 353
pure water since the cations of the alkaline boiler Table 17.7 Relationship between conductivity and salt fed
water additives (NH40H, NaOH) will be to the boiler
exchanged and recombine as
Conductivity Chloride in Salt going forward
+ OM- + H20 at 25°C condensate to boiler
(ps cm-') ( PPm) (g NaCl/Tonne)
A secondary advantage of such a system is the
enhancement of the conductivity due to replace- 0.137 0.01 0.0165
ment of cations by hydrogen ions which gives 0.604 0.05 0.0824
about a fivefold enhancement in ionic conduct- 1.200 0.10 0.1649
1.802
ance. This is particularly important with very 2.396 0.15 0.2473
0.3298
0.20
low leak rates. 6.003 0.50 0.8265
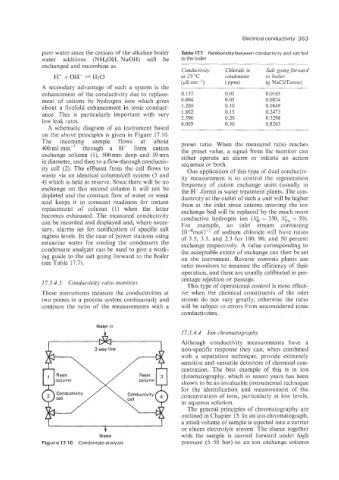
A schematic diagram of an instrument based
on the above principles is given in Figure 17.10.
The incoming sample flows at about preset ratio. When the measured ratio reaches
400ml min-* through a H+ form cation the preset value, a signal from the monitor can
exchange column (l), 500mm deep and 50mm either operate an alarm or initiate an action
in diameter, and then to a flow-through conductiv- sequence or both.
ity cell (2). The effluent from the cell flows to One application of this type of dual conductiv-
waste via an identical columnkell system (3 and ity measurement is to control the regeneration
4) which is held in reserve. Since there will be no frequency of cation exchange units (usually in
exchange on this second column it will not be the H+-form) in water treatment plants. The con-
depleted and the constant flow of water or weak ductivity at the outlet of such a unit will be higher
acid keeps it in constant readiness for instant than at the inlet since cations entering the ion
replacement of column (1) when the latter exchange bed will be replaced by the much more
becomes exhausted. The measured conductivity conductive hydrogen ion (A& = 350, A&, = 50).
can be recorded and displayed and, where neces- For example, an inlet stream containing
sary, alarms set for notification of specific salt 10-4mol I-' of sodium chloride will have ratios
ingress levels. In the case of power stations using of 3.5, 3.3, and 2.3 for 100, 90, and 50 percent
estuarine water for cooling the condensers the exchange respectively. A value corresponding to
condensate analyzer can be used to give a work- the acceptable extent of exchange can then be set
ing guide to the salt going forward to the boiler on the instrument. Reverse osmosis plants use
(see Table 17.7). ratio monitors to measure the efficiency of their
operation, and these are usually calibrated in per-
centage rejection or passage.
17.3.4.3 Conductivity ratio monitors
This type of operational control is most effect-
These instruments measure the conductivities at ive when the chemical constituents of the inlet
two points in a process system continuously and stream do not vary greatly; otherwise the ratio
compare the ratio of the measurements with a will be subject to errors from unconsidered ionic
conductivities.
Water in
1 17.3.4.4 Ion chromatography
Although conductivity measurements have a
non-specific response they can, when combined
with a separation technique, provide extremely
sensitive and versatile detectors of chemical con-
centration. The best example of this is in ion
chromatography, which in recent years has been
shown to be an invaluable instrumental technique
for the identification and measurement of the
concentration of ions, particularly at low levels,
in aqueous solution.
The general principles of chromatography are
outlined in Chapter 15. In an ion chromatograph,
a small volume of sample is injected into a carrier
1
or eluent electrolyte stream. The eluent together
Waste with the sample is carried forward under high
Figure 17.10 Condensate analyzer. pressure (5-50 bar) to an ion exchange column