Page 367 - Instrumentation Reference Book 3E
P. 367
350 Chemical analysis: electrochemical techniques
I signal Voltage control led
I L -
I I F generator
I I (fixed frequency)
electrode area, it can be appreciated that two- this type are free from the restrictions imposed
electrode conductivity cells have their limitations. by polarization. Therefore multiple-electrode
In circumstances where accurate measurements of cells can be standardized with any of the potas-
conductivity are required, in solutions of moder- sium chloride solutions given in Tables 17.4 and
ate or high conductivity or in solutions which can 17.5. The precaution previously stated about con-
readily contaminate the electrode surfaces, multi- stancy of temperature during any determination
ple-electrode cells should be considered. of cell constant must still be observed.
In its simplest form, a multiple-electrode cell Multiple-electrode cells are available with cell
has four electrodes in contact with the solution. constants from 0.1 to 10cm-' and can therefore
An outer pair operate similarly to those in a be used over a wide range of solution conductiv-
conventional two-electrode cell and an a.c. cur- ities. However, their most valuable applications are
rent is passed through the solution via these elec- when contamination or polarization is a problem.
trodes. The voltage drop across a segment of the
solution is measured potentiometrically at a sec-
ond or inner pair of the electrodes, and this drop Temperature compensation The conductivity of
will be proportional to the resistivity or inversely a solution is affected considerably by change of
proportional to the conductivity of the solution. temperatuse, and each solution has its own char-
Four-electrode cells can be operated in either the acteristic conductivity-temperature curve. Figure
constant-current or constant-voltage mode, but 17.7 shows how different these characteristics can
the latter is the more popular and will be be. When it is required to measure composition
described further. In this form of measurement rather than absolute conductivity it is therefore
the voltage at the inner electrode pair is main- essential to use a temperature compensator to
tained at a constant value by varying the current match the solution.
passed through the solution via the outer elec- Manual compensators consist of a variable and
trodes. The current flowing in the cell will be directly a fixed resistor in series. The temperature scale
proportional to the conductivity, and can be meas- showing the position of the contact on the variable
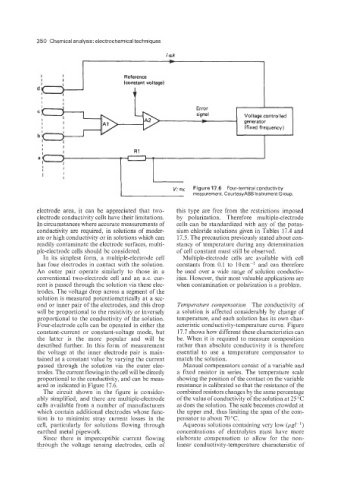
ured as indicated in Figure 17.6. resistance is calibrated so that the resistance of the
The circuit shown in the figure is consider- combined resistors changes by the same percentage
ably simplified, and there are multiple-electrode of the value of conductivity of the solution at 25 "C
cells available from a number of manufacturers as does the solution. The scale becomes crowded at
which contain additional electrodes whose func- the upper end, thus limiting the span of the com-
tion is to minimize stray current losses in the pensator to about 70°C.
cell, particularly for solutions flowing through Aqueous solutions containing very low (pgl-')
earthed metal pipework. concentrations of electrolytes must have more
Since there is imperceptible current flowing elaborate compensation to allow for the non-
through the voltage sensing electrodes, cells of linear conductivity-temperature characteristic of