Page 372 - Instrumentation Reference Book 3E
P. 372
The concept of pH 355
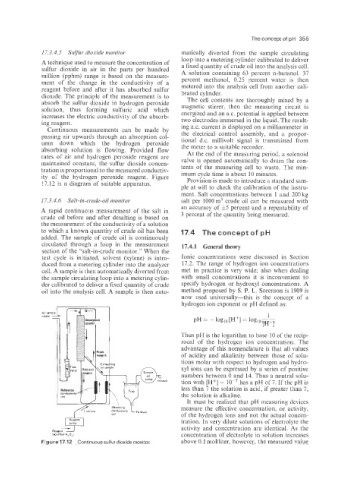
17.3.4.5 Sulfur dioxide monitor matically diverted from the sample circulating
A technique used to measure the concentration of loop into a metering cylinder calibrated to deliver
sulfur dioxide in air in the parts per hundred a fixed quantity of crude oil into the analysis cell.
A solution containing 63 percent n-butanol. 37
million (pphm) range is based on the measure-
ment of the change in the conductivity of a percent methanol, 0.25 percent water is then
reagent before and after it has absorbed sulfur metered into the analysis cell from another cali-
dioxide. The principle of the measurement is to brated cylinder.
absorb the sulfur dioxide in hydrogen peroxide The cell contents are thoroughly mixed by a
solution, thus forming sulfuric acid which magnetic stirrer, then the measuring circuit is
increases the electric conductivity of the absorb- energized and an ax. potential is applied between
two electrodes immersed in the liquid. The result-
ing reagent.
Continuous measurements can be made by ing a.c. current is displayed on a milliammeter in
passing air upwards through an absorption col- the electrical control assembly, and a propor-
tional d.c. millivolt signal is transmitted from
umn down which the hydrogen peroxide the meter to a suitable recorder.
absorbing solution is flowing. Provided flow
rates of air and hydrogen peroxide reagent are At the end of the measuring period, a solenoid
maintained constant: the sulfur dioxide concen- valve is opened automatically to drain the con-
tents of the measuring cell to waste. The min-
tration is proportional to the measured conductiv- imum cycle time is about 10 minutes.
ity of thLe hydrogen peroxide reagent. Figure
17.12 is a diagram of suitable apparatus. Provision is made to introduce a standard sam-
ple at will to check the calibration of the instru-
ment. Salt concentrations between 1 and 200kg
17.3.4.6 Salt-in-crude-oil monitor salt per iOOOm3 crude oil can be measured with
A rapid continuous measurement of the salt in an accuracy of ~t5 percent and a repeatability of
crude oil before and after desalting is based on 3 percent of the quantity being measured.
the measurement of the conductivity of a solution
to which a known quantity of crude oil has been 17.4 The concept of pH
added. The sample of crude oil is continuously
circulated through a loop in the measurement 17.4.1 General theory
section o F the “salt-in-crude monitor.” When the
test cycle is initiated, solvent (xylene) is intro- Ionic concentrations were discussed in Section
duced from a metering cylinder into the analyzer 17.2. The range of hydrogen ion Concentrations
cell. A sample is then automatically diverted from met in practice is very wide; also when dealing
the sample circulating loop into a metering cylin- with small concentrations it is inconvenient to
der calibrated to deliver a fixed quantity of crude specify hydrogen or hydroxyl concentrations. A
oil into the analysis cell. A sample is then auto- method proposed by S. P. L. Sorenson is 1909 is
now used universally-this is the concept of a
hydrogen ion exponent or pH defined as:
Airmrn
intake
Thus pH is the logarithm to base 10 of the recip-
rocal of the hydrogen ion concentration. The
advantage of this nomenclature is that all values
of acidity and alkalinity between those of soiu-
tions molar with respect to hydrogen and hydro-
xyl ions can be expressed by a series of positive
numbers between 0 and 14. Thus a neutral solu-
tion with [H+] = lop7 has a pH of 7. If the pW is
less than 7 the solution is acid, if greater than 7,
the solution is alkaline.
It must be realized that pH measuring devices
51 measure the effective concentration, or activity,
of the hydrogen ions and not the actual concen-
Reage”,
tration. In very dilute solutions of electrolyte the
activity and concentration are identical. As the
Reage”,
lacidifled H,02i concentration of electrolyte in solution increases
Figure 17.12 Continuous sulfur dioxide monitor. above 0.1 mollliter, however, the measured value