Page 381 - Instrumentation Reference Book 3E
P. 381
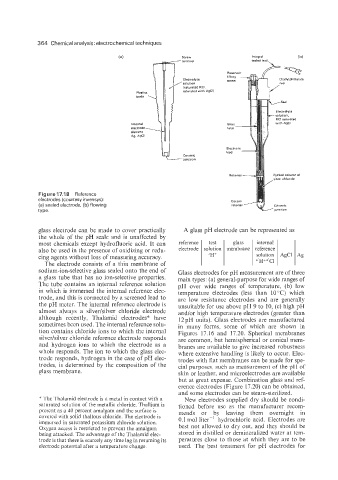
364 Chemical analysis: electrochemical techniques
(a) screw Integral
terminal sealed lead
Electrolyte Diallylohthalate
solution
lraturated KCI.
saturated wiih A9CI)
Internal
electrode
Ag. AgCl
G
Ceramic
-iunction
Packed column Of
Figure 17.1 8 Reference
electrodes (courtesy invensys):
(a) sealed electrode, (b) flowing
type.
glass electrode can be made to cover practically
the whole of the pH scale and is unaffected by
most chemicals except hydrofluoric acid. It can reference test glass internal
also be used in the presence of oxidizing or redu- electrode solution membrane reference
solution AgCl Ag
cing agents without loss of measuring accuracy. eIH+ d'H~d'C1
The electrode consists of a thin membrane of
sodium-ion-selective glass sealed onto the end of
a glass tube that has no ion-selective properties.
The tube contains an internal reference solution
in which is immersed the internal reference elec-
trode, and this is connected by a screened lead to
the pH meter. The internal reference electrode is
almost always a silver/silver chloride electrode
although recently, Thalamid electrodes* have
sometimes been used. The internal reference solu-
tion contains chloride ions to which the internal
silver/silver chloride reference electrode responds
and hydrogen ions to which the electrode as a
whole responds. The ion to which the glass elec-
trode responds, hydrogen in the case of pH elec-
trodes, is determined by the composition of the
glass membrane.
* The Thalamid electrode is a metal in contact with a
saturated solution of the metallic chloride. Thallium is
present as a 40 percent amalgam and the surface is
covered with solid thallous chloride. The electrode is
immersed in saturated potassium chloride solution.
Oxygen access is restricted to prevent the amalgam
being attacked. The advantage of the Thalamid elec-
trode is that there is scarcely any time lag in resuming its
electrode potential after a temperature change.