Page 529 - Instrumentation Reference Book 3E
P. 529
512 Optical measureme~ts
The author cannot r~conii~end this process.
Granted, it can be used for a magnificent
lecture-room Semons~ration- but in practice these
liquids are highly toxic, volatile, and have an
appalling smell. Moreover, the niethod depends
upon both the solid and the liquid being abso-
lutely colorless; if either has any trace of color it is I! \
difficult to judge the precise refractive index
match at which the boundaries disappear. -- -
---.-
Specific I ..
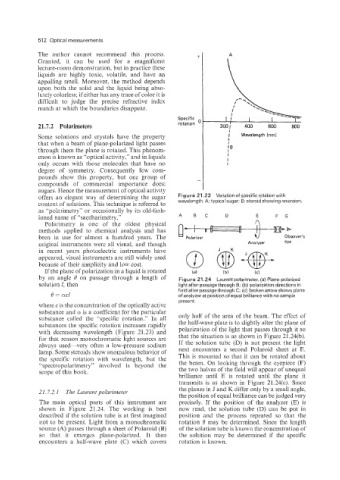
21.7.2 Polarimeters rotation O 200; 400 600 800
1 I Wavelength (nm)
Some solutions and crystals have the property I
that when a beam of plane-polarized light passes I
through them the piane is rotated. This phenom- I5
I
enon is known as “optical activity,” and in liquids I
only occws with those molecules that have no I i
degree of s~~e~ry. ~oiisequently few com-
pounds show this property, but one group of -
compounds of commercial importance does:
sugars. Hence the measurement of optical activity
offers an elegant way of determining the sugar Figure 21.23 Variation of specific rotation with
content of solutions. This technique is referred to wavelength. A: typical sugar: B: steroid showing reversion.
as “polarimetry” or occasionally by its old-fash-
ioned name of “saccharimetry.” A B C D E F G
Polarimetry is one of the oldest physical n n
1
I\
methods applied to chemical analysis and has 41 0 5 b
1
been in use for almost a hundred years. The €I* Polarizer Obrerver‘s
original instruments were all visual, and though Analyzer eye
in recent years photoelectric instruments have
appeared, visual instruments are still widely used
because of their simplicity and low cost.
If the plane of polarization in a liquid is rotated (ai (b) (Cl
by an angle 6 on PAssage through a fength of Figure 21.24 Laured ~ot~rimeter. Plane-poiari~ed
{a)
solution I, then light after passage through B; fb) polarization directions in
field after passage through C; (c) broken arrow shows plane
I9 = 0x1 of analyzer at position of equal bfllliance with no sample
present.
where G is the concentration of the optically active
substance and N is a coefficient for the particular
substance called the ”specific rotation.” In all only half of the area of the beam. The effect of
substances the specific rotation increases rapidly the half-wave plate is 10 slightly alter the plane of
with decreasing wavelength (Figure 21.23) and polarization of the light tliat passes through it so
for that reason monochromatic light sources are that the situation is as shown in Figure 21.24(b).
always used--very often a low-pressure sodium If the solution tube (D) is not present the light
lamp. Some steroids show anomalous behavior of next encounters a second Polaroid sheet at E.
the specific rotation with wavelength, but the This is mounted so that it can be rotated about
“spectropolarimetry” involved is beyond the the beam. On looking through the eyepiece (F)
scope of this book. the two halves of the field will appear of unequal
brilliance until E is rotated until the plane it
transmits is as shown in Figure 21.24jc). Since
the planes in J and K differ only by a sniall angle,
21.72.1 The Ltazcreflr ~ ~ ~ ~ r ~ ~ ? ~ ~ ~ ~ ~
the position of equal brilliance can be judged very
The main optical parts of this ui~~r~~eii~
are
precisely. If the position of the analyzer (E) is
shown in Figure 21.24. The working is best now read, the solution tube (D) cat1 be put in
described if the solution tube is at first imagined position and the process repeated so that the
not to be present. Light from a monochromatic rotation B may be determined. Since the length
source (A) passes through a sheet of Polaroid (B) of the solution tube is known the concentration of
so that it emerges plane-polarized. It then the solution may be determined if the specific
encounters a half-wave plate (C) which covers rotation is known.