Page 124 - Introduction to Computational Fluid Dynamics
P. 124
P1: IWV
CB908/Date
0521853265c04
EXERCISES
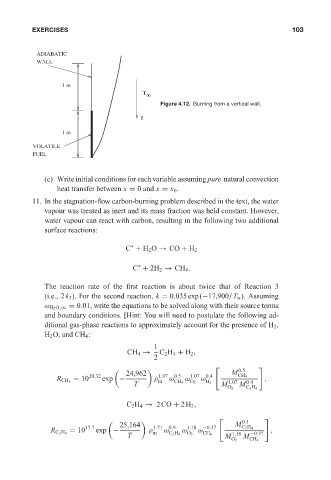
ADIABATIC 0 521 85326 5 May 25, 2005 11:7 103
WALL
1 m
T
8
Figure 4.12. Burning from a vertical wall.
g
1 m
VOLATILE
FUEL
(c) Write initial conditions for each variable assuming pure natural convection
heat transfer between x = 0 and x = x 0 .
11. In the stagnation-flow carbon-burning problem described in the text, the water
vapour was treated as inert and its mass fraction was held constant. However,
water vapour can react with carbon, resulting in the following two additional
surface reactions:
∗
C + H 2 O → CO + H 2
C + 2H 2 → CH 4 .
∗
The reaction rate of the first reaction is about twice that of Reaction 3
(i.e., 2k 3 ). For the second reaction, k = 0.035exp(−17,900/T w ). Assuming
ω H 2 O,∞ = 0.01, write the equations to be solved along with their source terms
and boundary conditions. [Hint: You will need to postulate the following ad-
ditional gas-phase reactions to approximately account for the presence of H 2 ,
H 2 O, and CH 4 :
1
CH 4 → C 2 H 4 + H 2 ,
2
24,962 M 0.5
= 10 20.32 exp − ρ 1.97 ω 0.5 ω 1.07 ω 0.4 CH 4 ,
R CH 4 m 1.07 0.4
T CH 4 O 2 H 2 M M
O 2 C 2 H 4
C 2 H 4 → 2CO + 2H 2 ,
0.1
25,164 −0.37 M
= 10 17.7 exp − ρ 1.71 ω 0.9 ω 1.18 ω C 2 H 4 ,
R C 2 H 4 m −0.37
T C 2 H 4 O 2 CH 4 M 1.18 M
O 2 CH 4