Page 36 - Introduction to Computational Fluid Dynamics
P. 36
P2: IWV
P1: JYD/GKJ
CB908/Date
0 521 85326 5
May 20, 2005
0521853265c01
EXERCISES
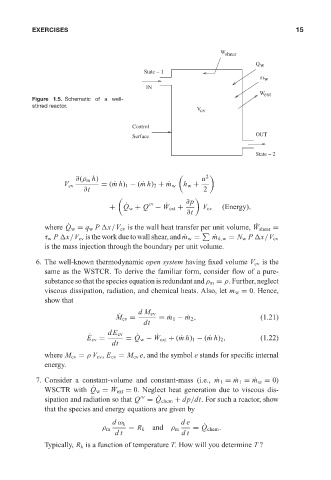
W shear 12:20 15
Q w
State -- 1
m w
IN
W ext
Figure 1.5. Schematic of a well-
stirred reactor.
V cv
Control
Surface OUT
State -- 2
∂(ρ m h) u 2
V cv = ( ˙ mh) 1 − ( ˙ mh) 2 + ˙ m w h w +
∂t 2
∂p
˙ ˙
+ Q w + Q − W ext + V cv (Energy),
∂t
˙ ˙
where Q w = q w P x/V cv is the wall heat transfer per unit volume, W shear =
τ w P x/V cv is the work due to wall shear, and ˙ m w = ˙ m k,w = N w P x/V cv
is the mass injection through the boundary per unit volume.
6. The well-known thermodynamic open system having fixed volume V cv is the
same as the WSTCR. To derive the familiar form, consider flow of a pure-
substance so that the species equation is redundant and ρ m = ρ. Further, neglect
viscous dissipation, radiation, and chemical heats. Also, let m w = 0. Hence,
show that
˙ dM cv
M cv = = ˙ m 1 − ˙ m 2 , (1.21)
dt
˙ dE cv ˙ ˙
E cv = = Q w − W ext + ( ˙ mh) 1 − ( ˙ mh) 2 , (1.22)
dt
where M cv = ρ V cv , E cv = M cv e, and the symbol e stands for specific internal
energy.
7. Consider a constant-volume and constant-mass (i.e., ˙ m 1 = ˙ m 1 = ˙ m w = 0)
˙
˙
WSCTR with Q w = W ext = 0. Neglect heat generation due to viscous dis-
˙
sipation and radiation so that Q = Q chem + dp/dt. For such a reactor, show
that the species and energy equations are given by
d ω k de ˙
ρ m = R k and ρ m = Q chem .
dt dt
Typically, R k is a function of temperature T. How will you determine T ?