Page 309 - Introduction to chemical reaction engineering and kinetics
P. 309
290 Chapter 11: Preliminary Considerations in Chemical Reaction Engineering
methanol synthesis must be selective (as well as active), to avoid formation of species
such as CH, and C,H,OH. The process was formerly carried out at high pressure (loo-
300 bar) with a ZnO/Cr,Os catalyst, but a catalyst of Cu/ZnO/Al,O, enables the reac-
tion to be carried out at 50-100 bar, which is referred to as low-pressure synthesis.
Figure 11.6 gives two examples of converters for low-pressure synthesis of methanol
with axial flow. Figure 11.6(a) shows a converter using heat exchanger surface (tube
cooling) within a single bed of catalyst. Figure 11.6(b) shows a converter using quench
cooling with quench gas added at three intervals in the bed through lozenge distributors.
11.2.4 Fluidized-Bed Reactors
In a fluidized-bed reactor, fluid is introduced at many points and at a sufficiently high
velocity that the upward flow through a bed of particles causes the particles to lift and
fall back in a recirculating pattern. The result is an expanded “fluidized-bed” of particles
+ fluid holdup, behaving somewhat like a vigorously boiling liquid. The fluid completes
its passage through the bed of particles by disengaging at the upper “surface.”
The fluidized-bed reactor was originally developed for catalytic “cracking” in petroleum
processing to enable continuous operation in a situation in which rapid fouling of cat-
alyst particles occurs. It may also be used for noncatalytic reactions in which the par-
ticulate material is a reactant. Kunii and Levenspiel (1991, Chapter 2) illustrate many
types of fluidized-bed reactors for various applications.
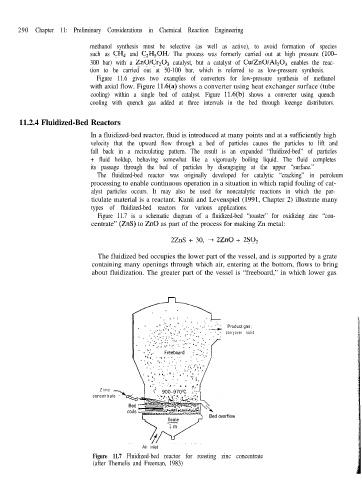
Figure 11.7 is a schematic diagram of a fluidized-bed “roaster” for oxidizing zinc “con-
centrate” (ZnS) to ZnO as part of the process for making Zn metal:
2ZnS + 30, + 2ZnO + 2S0,
The fluidized bed occupies the lower part of the vessel, and is supported by a grate
containing many openings through which air, entering at the bottom, flows to bring
about fluidization. The greater part of the vessel is “freeboard,” in which lower gas
. ._. . .I
1. _ . ‘, . ; - .,
Product gas;
carryover solid
Z i n c -
concentrate x
Air inlet
Figure 11.7 Fluidized-bed reactor for roasting zinc concentrate
(after Themelis and Freeman, 1983)