Page 305 - Introduction to chemical reaction engineering and kinetics
P. 305
286 Chapter 11: Preliminary Considerations in Chemical Reaction Engineering
11.2.3.1 Furnace Reactors
Some endothermic reactions require reactors that can provide high rates of heat trans-
fer at relatively high temperatures (900 to 1100 K). Examples are the dehydrogenation
of C,H6 to produce C,H, (noncatalytic, low P), and the steam-reforming of natural gas,
CH, + H,O -+ CO + 3H,, to produce Hz for ammonia and methanol syntheses (cat-
alytic, P = 30 bar). The reaction typically takes place as the reacting system (gas) flows
through long coils of tubes contained in a combustion chamber (furnace). Heat transfer
occurs by radiation and convection in corresponding sections of the reactor. A fuel gas
is burned in the combustion chamber. Figure 11.4 shows a schematic arrangement of a
“top-fired” furnace for steam reforming, in which the gas burners are located at the top
of the combustion chamber.
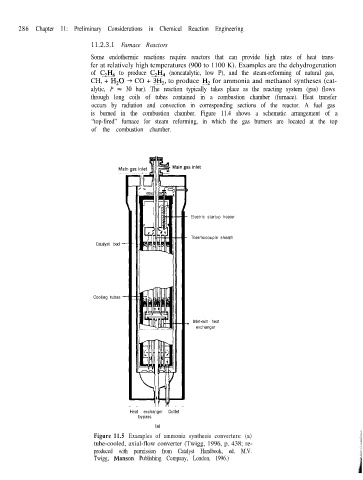
Electric startup heater
Thermocouple sheath
Catalyst bed
Cooling tubes
_ Inlet-exit heat
exchanger
Heat exchanger Outlet
bypass
(a)
Figure 11.5 Examples of ammonia synthesis converters: (a)
tube-cooled, axial-flow converter (Twigg, 1996, p. 438; re-
produced with permission from Catalyst Handbook, ed. M.V.
Twigg, Manson Publishing Company, London, 1996.)