Page 310 - Introduction to chemical reaction engineering and kinetics
P. 310
11.2 Examples of Reactors for Illustration of Process Design Considerations 291
velocity allows for partial disengagement of entrained particles from the overhead gas
stream. The solid reactant (zinc concentrate) enters at the left, and solid product leaves
at the right. Product gas, with some carryover solid, leaves at the top right (to proceed
to a cyclone for further removal of solid particles from the gas). The “coils” in the bed
are for a heat exchanger to control T.
Design aspects of fluidized-bed reactors are considered in Chapter 23.
11.2.5 Other Types of Reactors
There are many other reactors of various types not included among those discussed
above. These include tower reactors (Chapter 24), which may be modeled as PF or
modified PF reactors. We describe one further example in this section.
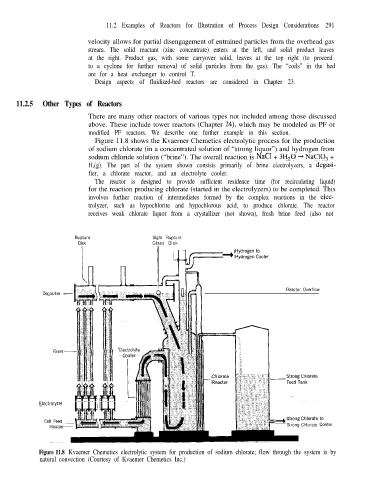
Figure 11.8 shows the Kvaerner Chemetics electrolytic process for the production
of sodium chlorate (in a concentrated solution of “strong liquor”) and hydrogen from
sodium chloride solution (“brine”). The overall reaction is NaCl + 3H,O + NaC103 +
H,(g). The part of the system shown consists primarily of brine electrolyzers, a degasi-
fier, a chlorate reactor, and an electrolyte cooler.
The reactor is designed to provide sufficient residence time (for recirculating liquid)
for the reaction producing chlorate (started in the electrolyzers) to be completed. This
involves further reaction of intermediates formed by the complex reactions in the elec-
trolyzer, such as hypochlorite and hypochlorous acid, to produce chlorate. The reactor
receives weak chlorate liquor from a crystallizer (not shown), fresh brine feed (also not
Rupture Sight Rupture
Disk Glass Disk
Degasifier - Reactor Overflow
Electrolyzel
Cell Feed
Strong Chlorate COI sler
Figure 11.8 Kvaemer Chemetics electrolytic system for production of sodium chlorate; flow through the system is by
natural convection (Courtesy of Kvaemer Chemetics Inc.)