Page 193 - Materials Science and Engineering An Introduction
P. 193
Questions and Problems • 165
Determine values for the activation energy and 5.35 (a) Calculate the diffusion coefficient for mag-
preexponential. nesium in aluminum at 450 C.
(b) What time will be required at 550 C to pro-
10 –15
duce the same diffusion result (in terms of con-
centration at a specific point) as for 15 h at 450 C?
5.36 A copper–nickel diffusion couple similar to that
shown in Figure 5.1a is fashioned. After a 500-h
heat treatment at 1000 C (1273 K), the concentra-
tion of Ni is 3.0 wt% at the 1.0-mm position within
the copper. At what temperature should the dif-
–16
fusion couple be heated to produce this same con-
Diffusion coefficient (m 2 /s) after 500 h? The preexponential and activation
10
centration (i.e., 3.0 wt% Ni) at a 2.0-mm position
4
energy for the diffusion of Ni in Cu are 1.9 10
2
m /s and 230,000 J/mol, respectively.
A diffusion couple similar to that shown in
metals A and B. After a 20-h heat treatment at
10 –17 5.37 Figure 5.1a is prepared using two hypothetical
800 C (and subsequently cooling to room tem-
perature) the concentration of B in A is 2.5 wt%
at the 5.0-mm position within metal A. If another
heat treatment is conducted on an identical diffu-
sion couple, but at 1000 C for 20 h, at what posi-
tion will the composition be 2.5 wt% B? Assume
that the preexponential and activation energy for
2
10 –18 the diffusion coefficient are 1.5 10 4 m /s and
0.49 0.50 0.51 0.52 0.53 0.54 0.55 0.56
Reciprocal temperature (1000/K) 125,000 J/mol, respectively.
5.38 Consider the diffusion of some hypothetical
metal Y into another hypothetical metal Z at
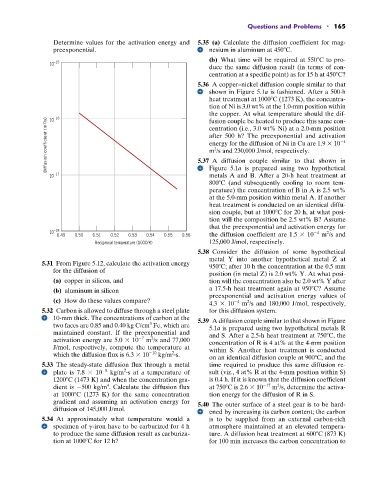
5.31 From Figure 5.12, calculate the activation energy 950 C; after 10 h the concentration at the 0.5 mm
for the diffusion of
position (in metal Z) is 2.0 wt% Y. At what posi-
(a) copper in silicon, and tion will the concentration also be 2.0 wt% Y after
(b) aluminum in silicon a 17.5-h heat treatment again at 950 C? Assume
preexponential and activation energy values of
(c) How do these values compare? 4.3 10 4 m /s and 180,000 J/mol, respectively,
2
5.32 Carbon is allowed to diffuse through a steel plate for this diffusion system.
10-mm thick. The concentrations of carbon at the 5.39 A diffusion couple similar to that shown in Figure
two faces are 0.85 and 0.40 kg C/cm Fe, which are 5.1a is prepared using two hypothetical metals R
3
maintained constant. If the preexponential and and S. After a 2.5-h heat treatment at 750 C, the
7
2
activation energy are 5.0 10 m /s and 77,000 concentration of R is 4 at% at the 4-mm position
J/mol, respectively, compute the temperature at within S. Another heat treatment is conducted
2
which the diffusion flux is 6.3 10 10 kg/m # s. on an identical diffusion couple at 900 C, and the
5.33 The steady-state diffusion flux through a metal time required to produce this same diffusion re-
plate is 7.8 10 8 kg/m # s at a temperature of sult (viz., 4 at% R at the 4-mm position within S)
2
1200 C (1473 K) and when the concentration gra- is 0.4 h. If it is known that the diffusion coefficient
2
4
dient is 500 kg/m . Calculate the diffusion flux at 750 C is 2.6 10 17 m /s, determine the activa-
at 1000 C (1273 K) for the same concentration tion energy for the diffusion of R in S.
gradient and assuming an activation energy for The outer surface of a steel gear is to be hard-
diffusion of 145,000 J/mol. 5.40 ened by increasing its carbon content; the carbon
5.34 At approximately what temperature would a is to be supplied from an external carbon-rich
specimen of g-iron have to be carburized for 4 h atmosphere maintained at an elevated tempera-
to produce the same diffusion result as carburiza- ture. A diffusion heat treatment at 600 C (873 K)
tion at 1000 C for 12 h? for 100 min increases the carbon concentration to