Page 339 - Materials Science and Engineering An Introduction
P. 339
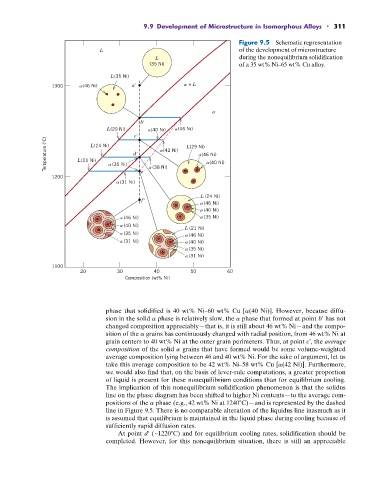
9.9 Development of Microstructure in Isomorphous Alloys • 311
Figure 9.5 Schematic representation
L of the development of microstructure
L during the nonequilibrium solidification
(35 Ni) of a 35 wt% Ni–65 wt% Cu alloy.
L (35 Ni)
1300 (46 Ni) a + L
b
L(29 Ni) (40 Ni) (46 Ni)
c
Temperature (°C) L(21 Ni) d (42 Ni) L(29 Ni) (40 Ni)
L(24 Ni)
(46 Ni)
(35 Ni)
e (38 Ni)
1200
(31 Ni)
L (24 Ni)
f
(46 Ni)
(40 Ni)
(46 Ni) (35 Ni)
(40 Ni)
L (21 Ni)
(35 Ni)
(46 Ni)
(31 Ni) (40 Ni)
(35 Ni)
(31 Ni)
1100
20 30 40 50 60
Composition (wt% Ni)
phase that solidified is 40 wt% Ni–60 wt% Cu [a(40 Ni)]. However, because diffu-
sion in the solid a phase is relatively slow, the a phase that formed at point b¿ has not
changed composition appreciably—that is, it is still about 46 wt% Ni—and the compo-
sition of the a grains has continuously changed with radial position, from 46 wt% Ni at
grain centers to 40 wt% Ni at the outer grain perimeters. Thus, at point c¿, the average
composition of the solid a grains that have formed would be some volume-weighted
average composition lying between 46 and 40 wt% Ni. For the sake of argument, let us
take this average composition to be 42 wt% Ni–58 wt% Cu [a(42 Ni)]. Furthermore,
we would also find that, on the basis of lever-rule computations, a greater proportion
of liquid is present for these nonequilibrium conditions than for equilibrium cooling.
The implication of this nonequilibrium solidification phenomenon is that the solidus
line on the phase diagram has been shifted to higher Ni contents—to the average com-
positions of the a phase (e.g., 42 wt% Ni at 1240 C)—and is represented by the dashed
line in Figure 9.5. There is no comparable alteration of the liquidus line inasmuch as it
is assumed that equilibrium is maintained in the liquid phase during cooling because of
sufficiently rapid diffusion rates.
At point d¿ (~1220 C) and for equilibrium cooling rates, solidification should be
completed. However, for this nonequilibrium situation, there is still an appreciable