Page 343 - Materials Science and Engineering An Introduction
P. 343
9.11 Binary Eutectic Systems • 315
Composition (at% Sn)
0 20 40 60 80 100
327°C
600
300
Liquid
500
232°C
+ L
Temperature (°C) 200 18.3 183°C 61.9 + L 97.8 400 Temperature (°F)
300
100
+
200
100
0
0 20 40 60 80 100
(Pb) Composition (wt% Sn) (Sn)
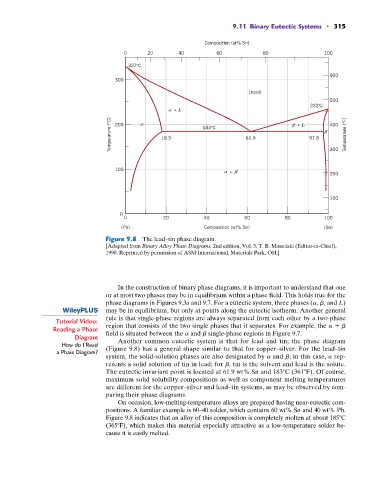
Figure 9.8 The lead–tin phase diagram.
[Adapted from Binary Alloy Phase Diagrams, 2nd edition, Vol. 3, T. B. Massalski (Editor-in-Chief),
1990. Reprinted by permission of ASM International, Materials Park, OH.]
In the construction of binary phase diagrams, it is important to understand that one
or at most two phases may be in equilibrium within a phase field. This holds true for the
phase diagrams in Figures 9.3a and 9.7. For a eutectic system, three phases (a, b, and L)
may be in equilibrium, but only at points along the eutectic isotherm. Another general
rule is that single-phase regions are always separated from each other by a two-phase
Tutorial Video:
Reading a Phase region that consists of the two single phases that it separates. For example, the a + b
field is situated between the a and b single-phase regions in Figure 9.7.
Diagram Another common eutectic system is that for lead and tin; the phase diagram
How do I Read (Figure 9.8) has a general shape similar to that for copper–silver. For the lead–tin
a Phase Diagram?
system, the solid-solution phases are also designated by a and b; in this case, a rep-
resents a solid solution of tin in lead; for b, tin is the solvent and lead is the solute.
The eutectic invariant point is located at 61.9 wt% Sn and 183 C (361 F). Of course,
maximum solid solubility compositions as well as component melting temperatures
are different for the copper–silver and lead–tin systems, as may be observed by com-
paring their phase diagrams.
On occasion, low-melting-temperature alloys are prepared having near-eutectic com-
positions. A familiar example is 60–40 solder, which contains 60 wt% Sn and 40 wt% Pb.
Figure 9.8 indicates that an alloy of this composition is completely molten at about 185 C
(365 F), which makes this material especially attractive as a low-temperature solder be-
cause it is easily melted.