Page 341 - Materials Science and Engineering An Introduction
P. 341
9.11 Binary Eutectic Systems • 313
60
60
400 50
Tensile strength (MPa) 300 50 Tensile strength (ksi) Elongation (% in 50 mm [2 in.]) 40
40
200 30 30
20
0 20 40 60 80 100 0 20 40 60 80 100
(Cu) (Ni) (Cu) (Ni)
Composition (wt% Ni) Composition (wt% Ni)
(a) (b)
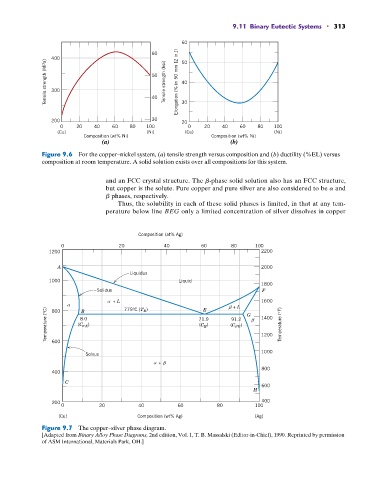
Figure 9.6 For the copper–nickel system, (a) tensile strength versus composition and (b) ductility (%EL) versus
composition at room temperature. A solid solution exists over all compositions for this system.
and an FCC crystal structure. The b-phase solid solution also has an FCC structure,
but copper is the solute. Pure copper and pure silver are also considered to be a and
b phases, respectively.
Thus, the solubility in each of these solid phases is limited, in that at any tem-
perature below line BEG only a limited concentration of silver dissolves in copper
Composition (at% Ag)
0 20 40 60 80 100
1200 2200
A 2000
Liquidus
1000 Liquid
1800
Solidus F
+ L 1600
B 779°C (T E ) E + L
Temperature (°C) (C ) 71.9 (C ) G 1400 Temperature (°F)
800
8.0
91.2
(C )
E
E
E
1200
600
1000
Solvus
+
800
400
C
600
H
200 400
0 20 40 60 80 100
(Cu) Composition (wt% Ag) (Ag)
Figure 9.7 The copper–silver phase diagram.
[Adapted from Binary Alloy Phase Diagrams, 2nd edition, Vol. 1, T. B. Massalski (Editor-in-Chief), 1990. Reprinted by permission
of ASM International, Materials Park, OH.]