Page 345 - Materials Science and Engineering An Introduction
P. 345
9.11 Binary Eutectic Systems • 317
600
300
Liquid
500
+ L
Temperature (°C) 200 B + L 400 Temperature (°F)
300
100 + 200
C
100
0
0 20 60 80 100
(Pb) (Sn)
C C 1
Composition (wt% Sn)
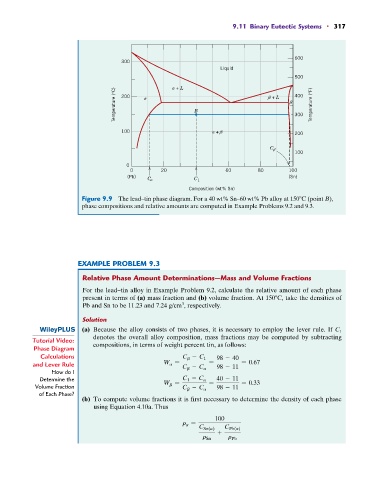
Figure 9.9 The lead–tin phase diagram. For a 40 wt% Sn–60 wt% Pb alloy at 150 C (point B),
phase compositions and relative amounts are computed in Example Problems 9.2 and 9.3.
EXAMPLE PROBLEM 9.3
Relative Phase Amount Determinations—Mass and Volume Fractions
For the lead–tin alloy in Example Problem 9.2, calculate the relative amount of each phase
present in terms of (a) mass fraction and (b) volume fraction. At 150 C, take the densities of
Pb and Sn to be 11.23 and 7.24 g/cm , respectively.
3
Solution
(a) Because the alloy consists of two phases, it is necessary to employ the lever rule. If C 1
denotes the overall alloy composition, mass fractions may be computed by subtracting
Tutorial Video: compositions, in terms of weight percent tin, as follows:
Phase Diagram
Calculations C b - C 1 98 - 40
and Lever Rule W a = = = 0.67
C b - C a 98 - 11
How do I
Determine the C 1 - C a 40 - 11
Volume Fraction W b = C b - C a = 98 - 11 = 0.33
of Each Phase?
(b) To compute volume fractions it is first necessary to determine the density of each phase
using Equation 4.10a. Thus
100
r a =
C Sn(a) C Pb(a)
+
r Sn r Pb