Page 235 - Materials Chemistry, Second Edition
P. 235
222 3 Metals
As temperature increases, the magnetic susceptibility, w, of a paramagnetic
substance decreases. The increasing thermal motion of atoms comprising the solid
disrupts the ordering among neighboring magnetic dipoles. Most often, the effective
magnetic moment, m eff , is used to describe the paramagnetic behavior, since this
quantity is independent of both the temperature and the magnitude of the external
field. Qualitatively, the macroscopic magnetic moment of a solid may be thought of
as a vector summation of all the microscopic magnetic dipole moments of each atom.
In a ferromagnetic material, the magnetic dipoles generated from unpaired
electrons tend to align in the same direction, even in the absence of an external
magnetic field. This phenomenon is aptly referred to as ferromagnetic coupling.
It should be noted that ferromagnetism is the direct opposite of superconductivity,
where all electron spins pair to form a perfectly diamagnetic material. Examples
of ferromagnetic behavior may be observed in bulk iron, cobalt, nickel, and some
rare earth elements (e.g., Gd). The regions containing parallel-aligned magnetic
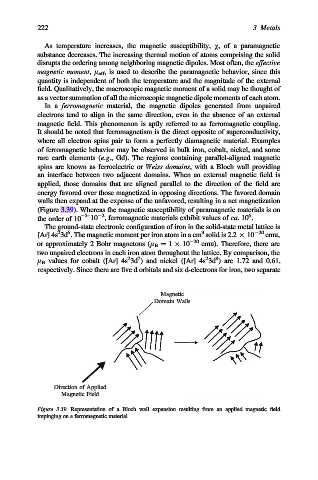
spins are known as ferroelectric or Weiss domains, with a Bloch wall providing
an interface between two adjacent domains. When an external magnetic field is
applied, those domains that are aligned parallel to the direction of the field are
energy favored over those magnetized in opposing directions. The favored domain
walls then expand at the expense of the unfavored, resulting in a net magnetization
(Figure 3.39). Whereas the magnetic susceptibility of paramagnetic materials is on
5– 2 6
the order of 10 10 , ferromagnetic materials exhibit values of ca. 10 .
The ground-state electronic configuration of iron in the solid-state metal lattice is
2 6 3 20
[Ar] 4s 3d . The magnetic moment per iron atom in a cm solid is 2.2 10 emu,
20
or approximately 2 Bohr magnetons (m B ¼ 1 10 emu). Therefore, there are
two unpaired electrons in each iron atom throughout the lattice. By comparison, the
8
2
7
2
m B values for cobalt ([Ar] 4s 3d ) and nickel ([Ar] 4s 3d ) are 1.72 and 0.61,
respectively. Since there are five d orbitals and six d-electrons for iron, two separate
Magnetic
Domain Walls
Direction of Applied
Magnetic Field
Figure 3.39. Representation of a Bloch wall expansion resulting from an applied magnetic field
impinging on a ferromagnetic material.