Page 237 - Materials Chemistry, Second Edition
P. 237
224 3 Metals
a saturation magnetization will again be found in the material, parallel to the new
direction of the applied field.
It is noteworthy that the bulk size and shape of a ferromagnetic metal may also
change as a result of reversible magnetization. This phenomenon, referred to as
magnetostriction, is due to the coupling of electron spins between neighboring
atoms, which affects the delocalized electrons involved in metallic bonding. This
effect is responsible for the familiar hum of transformers and fluorescent lights, due
to the vibration of the iron components within these materials.
At low temperatures, spontaneous antiferromagnetic coupling between neighbor-
ing atoms results in an equal number of magnetic dipoles in opposite directions.
However, as the temperature is increased, the dipoles are randomized resulting in
paramagnetic behavior. Primary examples of antiferromagnetic behavior include
transition metal compounds such as MnO, NiO, MnS, FeCO 3 , MnF 2 , as well as
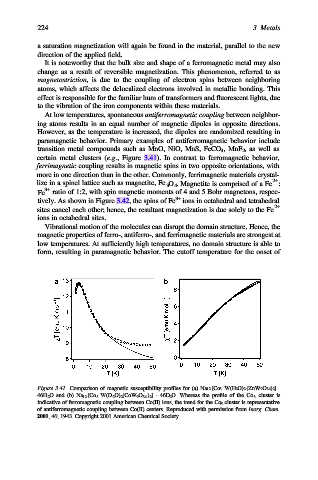
certain metal clusters (e.g., Figure 3.41). In contrast to ferromagnetic behavior,
ferrimagnetic coupling results in magnetic spins in two opposite orientations, with
more in one direction than in the other. Commonly, ferrimagnetic materials crystal-
2+
lize in a spinel lattice such as magnetite, Fe 3 O 4 . Magnetite is comprised of a Fe :
Fe 3+ ratio of 1:2, with spin magnetic moments of 4 and 5 Bohr magnetons, respec-
tively. As shown in Figure 3.42, the spins of Fe 3+ ions in octahedral and tetrahedral
2+
sites cancel each other; hence, the resultant magnetization is due solely to the Fe
ions in octahedral sites.
Vibrational motion of the molecules can disrupt the domain structure. Hence, the
magnetic properties of ferro-, antiferro-, and ferrimagnetic materials are strongest at
low temperatures. At sufficiently high temperatures, no domain structure is able to
form, resulting in paramagnetic behavior. The cutoff temperature for the onset of
Figure 3.41. Comparison of magnetic susceptibility profiles for (a) Na 12 [Co 3 W(H 2 O) 2 (ZnW 9 O 34 ) 2 ]·
46H 2 O and (b) Na 12 [Co 3 W(D 2 O) 2 (CoW 9 O 34 ) 2 ] · 46D 2 O. Whereas the profile of the Co 3 cluster is
indicative of ferromagnetic coupling between Co(II) ions, the trend for the Co 5 cluster is representative
of antiferromagnetic coupling between Co(II) centers. Reproduced with permission from Inorg. Chem.
2001, 40, 1943. Copyright 2001 American Chemical Society.