Page 234 - Materials Chemistry, Second Edition
P. 234
221
3.4. Magnetism
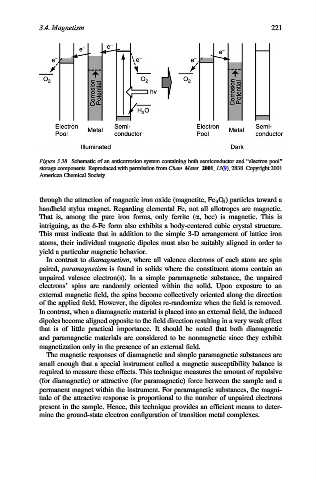
Figure 3.38. Schematic of an anticorrosion system containing both semiconductor and “electron pool”
storage components. Reproduced with permission from Chem. Mater. 2001, 13(9), 2838. Copyright 2001
American Chemical Society.
through the attraction of magnetic iron oxide (magnetite, Fe 3 O 4 ) particles toward a
handheld stylus magnet. Regarding elemental Fe, not all allotropes are magnetic.
That is, among the pure iron forms, only ferrite (a, bcc) is magnetic. This is
intriguing, as the d-Fe form also exhibits a body-centered cubic crystal structure.
This must indicate that in addition to the simple 3-D arrangement of lattice iron
atoms, their individual magnetic dipoles must also be suitably aligned in order to
yield a particular magnetic behavior.
In contrast to diamagnetism, where all valence electrons of each atom are spin
paired, paramagnetism is found in solids where the constituent atoms contain an
unpaired valence electron(s). In a simple paramagnetic substance, the unpaired
electrons’ spins are randomly oriented within the solid. Upon exposure to an
external magnetic field, the spins become collectively oriented along the direction
of the applied field. However, the dipoles re-randomize when the field is removed.
In contrast, when a diamagnetic material is placed into an external field, the induced
dipoles become aligned opposite to the field direction resulting in a very weak effect
that is of little practical importance. It should be noted that both diamagnetic
and paramagnetic materials are considered to be nonmagnetic since they exhibit
magnetization only in the presence of an external field.
The magnetic responses of diamagnetic and simple paramagnetic substances are
small enough that a special instrument called a magnetic susceptibility balance is
required to measure these effects. This technique measures the amount of repulsive
(for diamagnetic) or attractive (for paramagnetic) force between the sample and a
permanent magnet within the instrument. For paramagnetic substances, the magni-
tude of the attractive response is proportional to the number of unpaired electrons
present in the sample. Hence, this technique provides an efficient means to deter-
mine the ground-state electron configuration of transition metal complexes.