Page 19 - Mechanical Engineers' Handbook (Volume 4)
P. 19
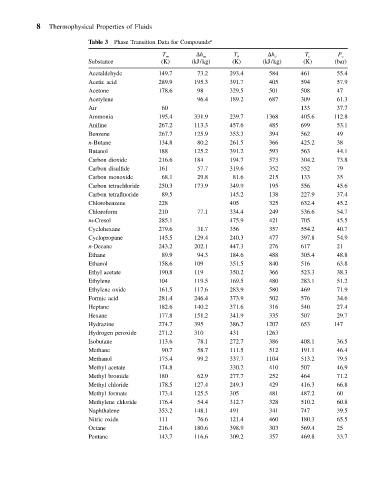
8 Thermophysical Properties of Fluids
Table 3 Phase Transition Data for Compounds a
T m h m T b h v T c P c
Substance (K) (kJ/kg) (K) (kJ/kg) (K) (bar)
Acetaldehyde 149.7 73.2 293.4 584 461 55.4
Acetic acid 289.9 195.3 391.7 405 594 57.9
Acetone 178.6 98 329.5 501 508 47
Acetylene 96.4 189.2 687 309 61.3
Air 60 133 37.7
Ammonia 195.4 331.9 239.7 1368 405.6 112.8
Aniline 267.2 113.3 457.6 485 699 53.1
Benzene 267.7 125.9 353.3 394 562 49
n-Butane 134.8 80.2 261.5 366 425.2 38
Butanol 188 125.2 391.2 593 563 44.1
Carbon dioxide 216.6 184 194.7 573 304.2 73.8
Carbon disulfide 161 57.7 319.6 352 552 79
Carbon monoxide 68.1 29.8 81.6 215 133 35
Carbon tetrachloride 250.3 173.9 349.9 195 556 45.6
Carbon tetrafluoride 89.5 145.2 138 227.9 37.4
Chlorobenzene 228 405 325 632.4 45.2
Chloroform 210 77.1 334.4 249 536.6 54.7
m-Cresol 285.1 475.9 421 705 45.5
Cyclohexane 279.6 31.7 356 357 554.2 40.7
Cyclopropane 145.5 129.4 240.3 477 397.8 54.9
n-Decane 243.2 202.1 447.3 276 617 21
Ethane 89.9 94.3 184.6 488 305.4 48.8
Ethanol 158.6 109 351.5 840 516 63.8
Ethyl acetate 190.8 119 350.2 366 523.3 38.3
Ethylene 104 119.5 169.5 480 283.1 51.2
Ethylene oxide 161.5 117.6 283.9 580 469 71.9
Formic acid 281.4 246.4 373.9 502 576 34.6
Heptane 182.6 140.2 371.6 316 540 27.4
Hexane 177.8 151.2 341.9 335 507 29.7
Hydrazine 274.7 395 386.7 1207 653 147
Hydrogen peroxide 271.2 310 431 1263
Isobutane 113.6 78.1 272.7 386 408.1 36.5
Methane 90.7 58.7 111.5 512 191.1 46.4
Methanol 175.4 99.2 337.7 1104 513.2 79.5
Methyl acetate 174.8 330.2 410 507 46.9
Methyl bromide 180 62.9 277.7 252 464 71.2
Methyl chloride 178.5 127.4 249.3 429 416.3 66.8
Methyl formate 173.4 125.5 305 481 487.2 60
Methylene chloride 176.4 54.4 312.7 328 510.2 60.8
Naphthalene 353.2 148.1 491 341 747 39.5
Nitric oxide 111 76.6 121.4 460 180.3 65.5
Octane 216.4 180.6 398.9 303 569.4 25
Pentane 143.7 116.6 309.2 357 469.8 33.7