Page 61 - Microsensors, MEMS and Smart Devices - Gardner Varadhan and Awadelkarim
P. 61
42 MEMS MATERIALS AND THEIR PREPARATION
3.1.2.1 Ionic bonding
In ionic bonding, one element gives up its outer-shell electron(s) to uncover a stable inner
shell of eight electrons (resembling the nearest noble element). The electrons are attracted
to a second element in which they can serve to complete its outer shell of eight (again
resembling the nearest noble element). An example of ionic bonding is the sodium chloride
(NaCl) molecule in which the sodium atom donates its 3s 1 electron, leaving an L shell of
5
2
eight (as in Ne), whereas the chlorine atom with an outer shell of seven (3s 3p ), attracts
that electron to form an outer shell of eight (as in AT). As a result of this electron-transfer
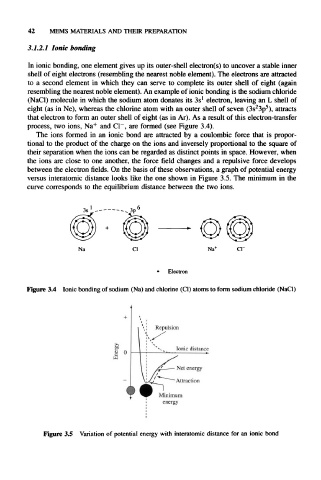
process, two ions, Na + and Cl~, are formed (see Figure 3.4).
The ions formed in an ionic bond are attracted by a coulombic force that is propor-
tional to the product of the charge on the ions and inversely proportional to the square of
their separation when the ions can be regarded as distinct points in space. However, when
the ions are close to one another, the force field changes and a repulsive force develops
between the electron fields. On the basis of these observations, a graph of potential energy
versus interatomic distance looks like the one shown in Figure 3.5. The minimum in the
curve corresponds to the equilibrium distance between the two ions.
cl Na + cl
• Electron
Figure 3.4 Ionic bonding of sodium (Na) and chlorine (Cl) atoms to form sodium chloride (NaCl)
Repulsion
Attraction
Minimum
energy
Figure 3.5 Variation of potential energy with interatomic distance for an ionic bond