Page 66 - Microsensors, MEMS and Smart Devices - Gardner Varadhan and Awadelkarim
P. 66
OVERVIEW 47
SC structure, each atom or a group of atoms at some lattice point is surrounded by six
nearest neighbours.
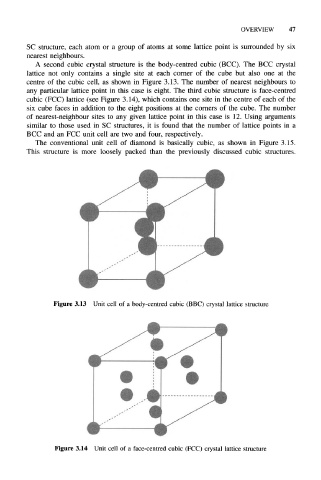
A second cubic crystal structure is the body-centred cubic (BCC). The BCC crystal
lattice not only contains a single site at each corner of the cube but also one at the
centre of the cubic cell, as shown in Figure 3.13. The number of nearest neighbours to
any particular lattice point in this case is eight. The third cubic structure is face-centred
cubic (FCC) lattice (see Figure 3.14), which contains one site in the centre of each of the
six cube faces in addition to the eight positions at the comers of the cube. The number
of nearest-neighbour sites to any given lattice point in this case is 12. Using arguments
similar to those used in SC structures, it is found that the number of lattice points in a
BCC and an FCC unit cell are two and four, respectively.
The conventional unit cell of diamond is basically cubic, as shown in Figure 3.15.
This structure is more loosely packed than the previously discussed cubic structures.
Figure 3.13 Unit cell of a body-centred cubic (BBC) crystal lattice structure
Figure 3.14 Unit cell of a face-centred cubic (FCC) crystal lattice structure