Page 62 - Microsensors, MEMS and Smart Devices - Gardner Varadhan and Awadelkarim
P. 62
OVERVIEW 43
3.1.2.2 Covalent bonding
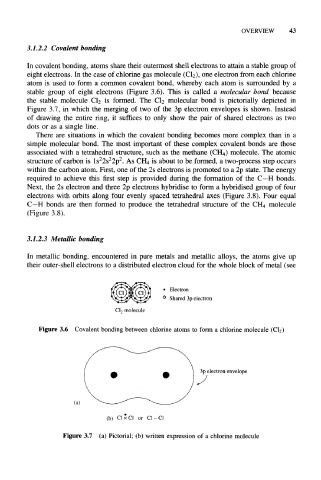
In covalent bonding, atoms share their outermost shell electrons to attain a stable group of
eight electrons. In the case of chlorine gas molecule (Cl 2), one electron from each chlorine
atom is used to form a common covalent bond, whereby each atom is surrounded by a
stable group of eight electrons (Figure 3.6). This is called a molecular bond because
the stable molecule Cl 2 is formed. The Cl 2 molecular bond is pictorially depicted in
Figure 3.7, in which the merging of two of the 3p electron envelopes is shown. Instead
of drawing the entire ring, it suffices to only show the pair of shared electrons as two
dots or as a single line.
There are situations in which the covalent bonding becomes more complex than in a
simple molecular bond. The most important of these complex covalent bonds are those
associated with a tetrahedral structure, such as the methane (CH 4) molecule. The atomic
2 2 2
structure of carbon is Is 2s 2p . As CH4 is about to be formed, a two-process step occurs
within the carbon atom. First, one of the 2s electrons is promoted to a 2p state. The energy
required to achieve this first step is provided during the formation of the C—H bonds.
Next, the 2s electron and three 2p electrons hybridise to form a hybridised group of four
electrons with orbits along four evenly spaced tetrahedral axes (Figure 3.8). Four equal
C—H bonds are then formed to produce the tetrahedral structure of the CH 4 molecule
(Figure 3.8).
3.1.2.3 Metallic bonding
In metallic bonding, encountered in pure metals and metallic alloys, the atoms give up
their outer-shell electrons to a distributed electron cloud for the whole block of metal (see
• Electron
° Shared 3p electron
C1 2 molecule
Figure 3.6 Covalent bonding between chlorine atoms to form a chlorine molecule
3p electron envelope
(a)
(b) ClxCl or C1-C1
Figure 3.7 (a) Pictorial; (b) written expression of a chlorine molecule