Page 63 - Microsensors, MEMS and Smart Devices - Gardner Varadhan and Awadelkarim
P. 63
44 MEMS MATERIALS AND THEIR PREPARATION
Covalentbond
Carbon atom
Hydrogen atom
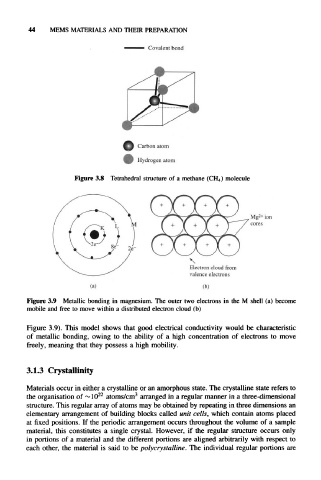
Figure 3.8 Tetrahedral structure of a methane (CH 4) molecule
2+
Mg ion
cores
\
Electron cloud from
valence electrons
(a) (b)
Figure 3.9 Metallic bonding in magnesium. The outer two electrons in the M shell (a) become
mobile and free to move within a distributed electron cloud (b)
Figure 3.9). This model shows that good electrical conductivity would be characteristic
of metallic bonding, owing to the ability of a high concentration of electrons to move
freely, meaning that they possess a high mobility.
3.1.3 Crystallinity
Materials occur in either a crystalline or an amorphous state. The crystalline state refers to
3
the organisation of ~10 22 atoms/cm arranged in a regular manner in a three-dimensional
structure. This regular array of atoms may be obtained by repeating in three dimensions an
elementary arrangement of building blocks called unit cells, which contain atoms placed
at fixed positions. If the periodic arrangement occurs throughout the volume of a sample
material, this constitutes a single crystal. However, if the regular structure occurs only
in portions of a material and the different portions are aligned arbitrarily with respect to
each other, the material is said to be polycrystalline. The individual regular portions are