Page 300 - Modern Analytical Chemistry
P. 300
1400-CH09 9/9/99 2:12 PM Page 283
Chapter 9 Titrimetric Methods of Analysis 283
–
–
CH 3 COO (aq)+H 2 O(l) t OH (aq)+CH 3 COOH(aq)
-
xx
[ OH ][ CH 3 COOH] ()() - 10
K b = = = .571 ´ 10
-
[CH 3 COO ] . 0 0500 - x
–6
–
x = [OH ] = 5.34 ´10 M
+
–9
The concentration of H 3 O , therefore, is 1.87 ´10 , or a pH of 8.73.
After the equivalence point NaOH is present in excess, and the pH is deter-
mined in the same manner as in the titration of a strong acid with a strong base. For
–
example, after adding 60.0 mL of NaOH, the concentration of OH is
)
0
0
60
(.100 M )( . mL - (.100 M )( . mL )
0
50
0
[OH - ] = = . 0 00909 M
0
0
50 . mL +60 . mL
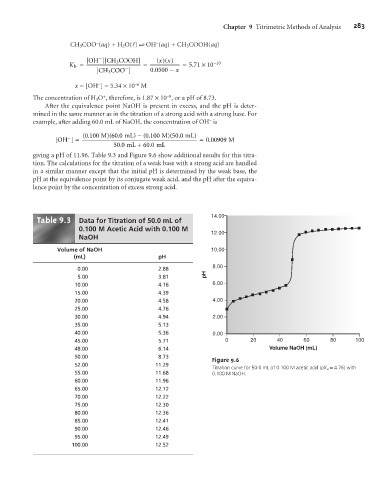
giving a pH of 11.96. Table 9.3 and Figure 9.6 show additional results for this titra-
tion. The calculations for the titration of a weak base with a strong acid are handled
in a similar manner except that the initial pH is determined by the weak base, the
pH at the equivalence point by its conjugate weak acid, and the pH after the equiva-
lence point by the concentration of excess strong acid.
9 3
Table . Data for Titration of 50.0 mL of 14.00
0.100 M Acetic Acid with 0.100 M
12.00
NaOH
Volume of NaOH 10.00
(mL) pH
0.00 2.88 8.00
5.00 3.81 pH
10.00 4.16 6.00
15.00 4.39
20.00 4.58 4.00
25.00 4.76
30.00 4.94 2.00
35.00 5.13
40.00 5.36 0.00
45.00 5.71 0 20 40 60 80 100
48.00 6.14 Volume NaOH (mL)
50.00 8.73 Figure 9.6
52.00 11.29
Titration curve for 50.0 mL of 0.100 M acetic acid (pK a = 4.76) with
55.00 11.68 0.100 M NaOH.
60.00 11.96
65.00 12.12
70.00 12.22
75.00 12.30
80.00 12.36
85.00 12.41
90.00 12.46
95.00 12.49
100.00 12.52