Page 199 - Modern physical chemistry
P. 199
B.lO Debye-HiickeJ Equations 191
or
2
logy j = -Azj .j;. [8.96J
In equation (8.96), all the constants have been gathered together into expressionA. This
equation is known as the Debye-Hilckellimiting law.
In actual systems, the ionic atmosphere about an ion can build up only in the region
outside the ion. A large ion has a larger excluded volume than a smaller one and hence
less of an ionic atmosphere, other things being equal. So it would have a larger activity
coefficient in a solution of given ionic strength.
The difference is embodied in parameter a j in formula (8.87). Employing (8.87) instead
of (8.83) leads to the equation
AZ.2 r;;
logy j = _ J '\j J1. • [8.97J
1 + Baj.j;
Here rj. is the activity coefficient of ion j, Zj the number of positive charges on j, as
employed in equation (8.47), a j the effective diameter of ion j in solution, 11 the ionic
strength, while A, B are constants depending on the temperature and solvent. This result
is the single ion Debye-Hilckel equation.
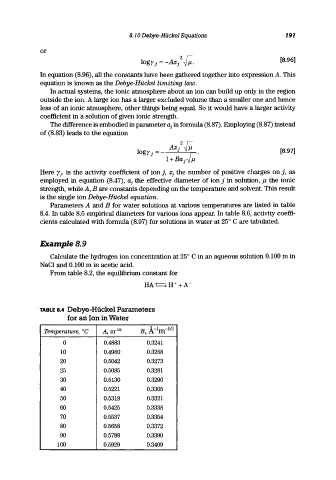
Parameters A and B for water solutions at various temperatures are listed in table
8.4. In table 8.5 empirical diameters for various ions appear. In table 8.6, activity coeffi-
cients calculated with formula (8.97) for solutions in water at 25° C are tabulated.
ExampleB.9
Calculate the hydrogen ion concentration at 25° C in an aqueous solution 0.100 m in
NaCI and 0.100 m in acetic acid.
From table 8.2, the equilibrium constant for
HA ( ) H+ +A-
TABLE 8.4 Debye-Hiickel Parameters
for an Ion in Water
Temperature, °C A, m- l12 B, A- 1 rn- 1I2
0 0.4883 0.3241
10 0.4960 0.3258
20 0.5042 0.3273
25 0.5085 0.3281
30 0.5130 0.3290
40 0.5221 0.3305
50 0.5319 0.3321
60 0.5425 0.3338
70 0.5537 0.3354
80 0.5658 0.3372
90 0.5788 0.3390
100 0.5929 0.3409