Page 201 - Modern physical chemistry
P. 201
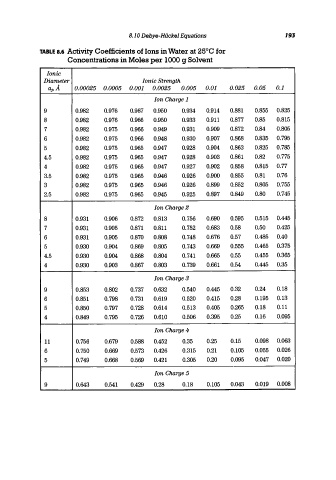
8.10 Debye-HiickeJ Equations 193
TABLE 8.6 Activity Coefficients of Ions in Water at 25°C for
Concentrations in Moles per 1000 g Solvent
Ianie
Diameter Ionic Strength
aj,A 0.00025 0.0005 0.001 0.0025 0.005 0.01 0.025 0.05 0.1
Ion Charge 1
9 0.982 0.976 0.967 0.950 0.934 0.914 0.881 0.855 0.825
8 0.982 0.976 0.966 0.950 0.933 0.911 0.877 0.85 0.815
7 0.982 0.975 0.966 0.949 0.931 0.909 0.872 0.84 0.805
6 0.982 0.975 0.966 0.948 0.930 0.907 0.868 0.835 0.795
5 0.982 0.975 0.965 0.947 0.928 0.904 0.863 0.825 0.785
4.5 0.982 0.975 0.965 0.947 0.928 0.903 0.861 0.82 0.775
4 0.982 0.975 0.965 0.947 0.927 0.902 0.858 0.815 0.77
3.5 0.982 0.975 0.965 0.946 0.926 0.900 0.855 0.81 0.76
3 0.982 0.975 0.965 0.946 0.926 0.899 0.852 0.805 0.755
2.5 0.982 0.975 0.965 0.945 0.925 0.897 0.849 0.80 0.745
Ion Charge 2
8 0.931 0.906 0.872 0.813 0.756 0.690 0.595 0.515 0.445
7 0.931 0.905 0.871 0.811 0.752 0.683 0.58 0.50 0.425
6 0.931 0.905 0.870 0.808 0.748 0.676 0.57 0.485 0.40
5 0.930 0.904 0.869 0.805 0.743 0.669 0.555 0.465 0.375
4.5 0.930 0.904 0.868 0.804 0.741 0.665 0.55 0.455 0.365
4 0.930 0.903 0.867 0.803 0.739 0.661 0.54 0.445 0.35
Ian Charge 3
9 0.853 0.802 0.737 0.632 0.540 0.445 0.32 0.24 0.18
6 0.851 0.798 0.731 0.619 0.520 0.415 0.28 0.195 0.13
5 0.850 0.797 0.728 0.614 0.513 0.405 0.265 0.18 0.11
4 0.849 0.795 0.726 0.610 0.506 0.395 0.25 0.16 0.095
Ion Charge 4
11 0.756 0.679 0.588 0.452 0.35 0.25 0.15 0.098 0.063
6 0.750 0.669 0.573 0.426 0.315 0.21 0.105 0.055 0.026
5 0.749 0.668 0.569 0.421 0.305 0.20 0.095 0.047 0.020
Ion Charge 5
9 0.643 0.541 0.429 0.28 0.18 0.105 0.043 0.019 0.008