Page 217 - Modern physical chemistry
P. 217
210 Electrochemistry
reactions occur at the surface of each electrode, depleting some ions and augmenting
others. As a result the electrolyte becomes more dilute near one electrode and more con-
centrated near the other. Associated with the flow of charge is a transport of the electrolyte.
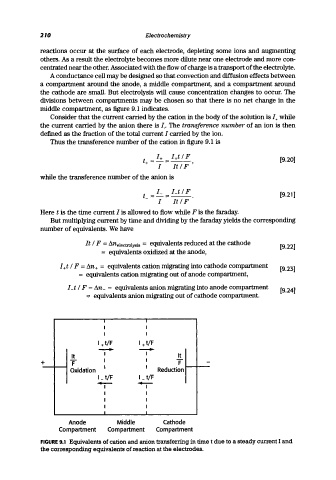
A conductance cell may be designed so that convection and diffusion effects between
a compartment around the anode, a middle compartment, and a compartment around
the cathode are small. But electrolysis will cause concentration changes to occur. The
divisions between compartments may be chosen so that there is no net change in the
middle compartment, as figure 9.1 indicates.
Consider that the current carried by the cation in the body of the solution is 1+ while
the current carried by the anion there is L The transference number of an ion is then
defined as the fraction of the total current I carried by the ion.
Thus the transference number of the cation in figure 9.1 is
t _ 1+ _ I+t IF [9.20]
+ - I - It IF'
while the transference number of the anion is
L = L = Ltl F. [9.21 ]
I It IF
Here t is the time current I is allowed to flow while F is the faraday.
But multiplying current by time and dividing by the faraday yields the corresponding
number of equivalents. We have
It I F = Anelectrolysis = equivalents reduced at the cathode [9.22]
= equivalents oxidized at the anode,
I +t I F = An+ = equivalents cation migrating into cathode compartment [9.23]
= equivalents cation migrating out of anode compartment,
I _t I F = An_ = equivalents anion migrating into anode compartment [9.24]
= equivalents anion migrating out of cathode compartment.
I I
I I I I
It - -
1+ t/F
I +t/F
I
+ F I I I It -
F
- -
Oxidation I I Reduction
L t/F
L t/F
I I I I
I I
I I
Anode Middle Cathode
Compartment Compartment Compartment
FIGURE 9.1 Equivalents of cation and anion transferring in time t due to a steady current I and
the corresponding equivalents of reaction at the electrodes.