Page 218 - Modern physical chemistry
P. 218
9.3 Electrolyte Transference 211
In practice, the compartments may be separated by porous membranes. Or better,
the electrolysis tube may be shaped to keep the contents of the compartments separate.
For a run, electrolyte of known concentration is placed in the tube. Current is passed
through for an interval of time and the number of faradays determined with a coulome-
ter in series with the cell. About each electrode, all solution that differs from that in the
middle compartment is removed and analyzed. The amount of electrolyte originally
present in the water is calculated. The change in equivalents is then
[9.25]
for each ion.
For one ion and one compartment, the equivalents released at the electrode are Llne1ec_
trolysis' The equivalents migrating are then
[9.26]
Llnmigration = Lln - LlnelectrolysiS.
The absolute value of Ll~tion divided by Llnelectrolysis equals the transference number of
the ion.
This method was developed by J. W. Hittorf. For accurate work, one should correct for
the water transported with the ions. Alternatively, one may employ the moving boundary
method discussed in section 9.4. Or, the voltage of an electrolytic cell with transference
can be compared to the same cell without transference.
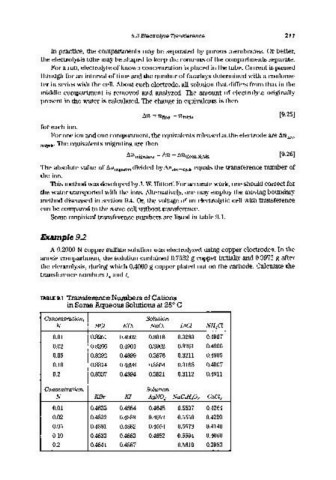
Some empirical transference numbers are listed in table 9.1.
ExampJe9.2
A 0.2000 N copper sulfate solution was electrolyzed using copper electrodes. In the
anode compartment, the solution contained 0.7532 g copper initially and 0.9972 g after
the electrolysis, during which 0.4000 g copper plated out on the cathode. Calculate the
transference numbers t+ and L
TABLE 9.1 Transference Nwnbers of Cations
in Some Aqueous Solutions at 25 C
0
Concentration, Solution
N HCl KCl NaCl LiCl NH4Gl
0,01 0.8251 0.4902 0.3918 0.3289 0.4907
0.02 0.8266 0.4901 0.3902 0.3261 0.4906
0.05 0.8292 0.4899 0.3876 0.3211 0.4905
0.10 0.8314 0.4898 0.3854 0.3168 0.4907
0.2 0.8337 0.4894 0.3821 0.3112 0.4911
Concentration, Solution
N KBr KI AgN0 3 NaCtP30 2 CaGl2
0.01 0.4833 0.4884 0.4648 0.5537 0.4264
0.02 0.4832 0.4883 0.4652 0.5550 0.4220
0.05 0.4831 0.4882 0.4664 0.5573 0.4140
0.10 0.4833 0.4883 0.4682 0.5594 0.4060
0.2 0.4841 0.4887 - 0.5610 0.3953