Page 52 - Multifunctional Photocatalytic Materials for Energy
P. 52
Metal oxide electrodes for photo-activated water splitting 41
(A) (B)
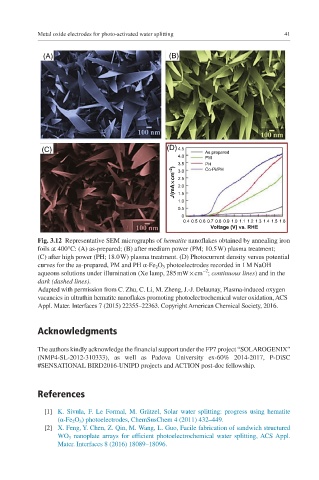
(C) (D) 4.5 As prepared
4.0 PM
J (mA¥ cm –2 ) 3.5 Co-Pi/PH
PH
3.0
2.5
2.0
1.5
1.0
0.5
0
0.4 0.5 0.6 0.7 0.8 0.9 1.0 1.1 1.2 1.3 1.4 1.5 1.6
Voltage (V) vs. RHE
Fig. 3.12 Representative SEM micrographs of hematite nanoflakes obtained by annealing iron
foils at 400°C: (A) as-prepared; (B) after medium power (PM; 10.5 W) plasma treatment;
(C) after high power (PH; 18.0 W) plasma treatment. (D) Photocurrent density versus potential
curves for the as-prepared, PM and PH α-Fe 2 O 3 photoelectrodes recorded in 1 M NaOH
−2
aqueous solutions under illumination (Xe lamp, 285 mW × cm ; continuous lines) and in the
dark (dashed lines).
Adapted with permission from C. Zhu, C. Li, M. Zheng, J.-J. Delaunay, Plasma-induced oxygen
vacancies in ultrathin hematite nanoflakes promoting photoelectrochemical water oxidation, ACS
Appl. Mater. Interfaces 7 (2015) 22355–22363. Copyright American Chemical Society, 2016.
Acknowledgments
The authors kindly acknowledge the financial support under the FP7 project “SOLAROGENIX”
(NMP4-SL-2012-310333), as well as Padova University ex-60% 2014-2017, P-DiSC
#SENSATIONAL BIRD2016-UNIPD projects and ACTION post-doc fellowship.
References
[1] K. Sivula, F. Le Formal, M. Grätzel, Solar water splitting: progress using hematite
(α-Fe 2 O 3 ) photoelectrodes, ChemSusChem 4 (2011) 432–449.
[2] X. Feng, Y. Chen, Z. Qin, M. Wang, L. Guo, Facile fabrication of sandwich structured
WO 3 nanoplate arrays for efficient photoelectrochemical water splitting, ACS Appl.
Mater. Interfaces 8 (2016) 18089–18096.