Page 101 - Book Hosokawa Nanoparticle Technology Handbook
P. 101
2.3 PARTICLE SHAPE FUNDAMENTALS
key points for controlling the shape and size of described below. It is also effective to use the template
nanoparticles are described. method as described above.
When the supplied monomers are consumed at a
(2) Shape and size control of nanoparticles nucleus growth rate that is faster than the nucleation
When generated particles are amorphous, two- rate, the crystalline particles become anisotropic. The
dimensional nucleation does not occur on the parti- nucleus growth is classified into diffusion and surface
cle surface. Thus, the generated nuclei can grow in reaction controlled-growth by the balance between
any direction at the same growth rate, and form the diffusion coefficient of monomers and the reac-
spherical particles. The typical example of this type tion rate on the crystal face. The former shape is
of particle synthesis is the sol-gel method. In this determined by the differences between the surface
case, the template method controls the shape and energies of the crystal faces, and the latter shape is
size of spherical amorphous particles satisfactorily. determined by the reaction rate at each crystal face.
For example, the surfactant in the solvent forms a In this case, the shape and size of nanoparticles can
hexagonal structure and a lamellar structure, etc., be controlled if the growth of the crystal face is sup-
depending on the concentration. Using these struc- pressed by an additive that is selectively adsorbed to a
tures as templates, rod-like particles, plate particles, specific crystal face. Additives used for suppressing
etc., can be synthesized. the growth are anions, amines, carboxylic acids, sur-
When the generated particles are crystalline, it is factants, etc.
important to keep a balance between the nucleation and As a specific example in which the shape and size
the growth rates of nuclei. If the nucleation rate is faster of nanocrystalline particles are controlled, the
than the growth rate, the generated particles form poly- microemulsion method using the interfacial activities
crystalline spherical particles. In the case of sulfide and of reverse micelles and microemulsions was intro-
oxide particles, the critical supersaturation concentra- duced [5]. Barium chromate was used as the model
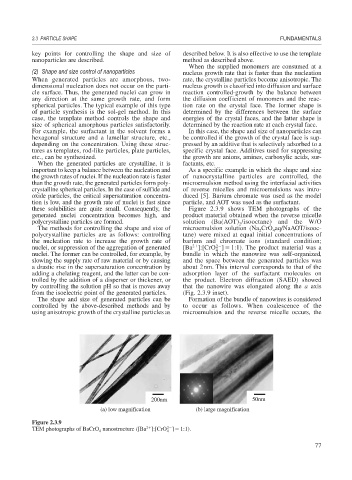
tion is low, and the growth rate of nuclei is fast since particle, and AOT was used as the surfactant.
these solubilities are quite small. Consequently, the Figure 2.3.9 shows TEM photographs of the
generated nuclei concentration becomes high, and product material obtained when the reverse micelle
polycrystalline particles are formed. solution (Ba(AOT) /isooctane) and the W/O
2
The methods for controlling the shape and size of microemulsion solution (Na CrO aq/NaAOT/isooc-
4
2
polycrystalline particles are as follows: controlling tane) were mixed at equal initial concentrations of
the nucleation rate to increase the growth rate of barium and chromate ions (standard condition;
2
2
nuclei, or suppression of the aggregation of generated [Ba ]:[CrO ] 1:1). The product material was a
4
nuclei. The former can be controlled, for example, by bundle in which the nanowire was self-organized,
slowing the supply rate of raw material or by causing and the space between the generated particles was
a drastic rise in the supersaturation concentration by about 2nm. This interval corresponds to that of the
adding a chelating reagent, and the latter can be con- adsorption layer of the surfactant molecules on
trolled by the addition of a disperser or thickener, or the product. Electron diffraction (SAED) showed
by controlling the solution pH so that is moves away that the nanowire was elongated along the a axis
from the isoelectric point of the generated particles. (Fig. 2.3.9 inset).
The shape and size of generated particles can be Formation of the bundle of nanowires is considered
controlled by the above-described methods and by to occur as follows. When coalescence of the
using anisotropic growth of the crystalline particles as microemulsion and the reverse micelle occurs, the
200nm 50nm
(a) low magnification (b) large magnification
Figure 2.3.9
2
2
TEM photographs of BaCrO nanostructure ([Ba ]:[CrO ] 1:1).
4
4
77