Page 98 - Book Hosokawa Nanoparticle Technology Handbook
P. 98
FUNDAMENTALS CH. 2 STRUCTURAL CONTROL OF NANOPARTICLES
T=293K T=1073K
200 nm 200 nm
T=1273K T=1573K
200 nm 200 nm
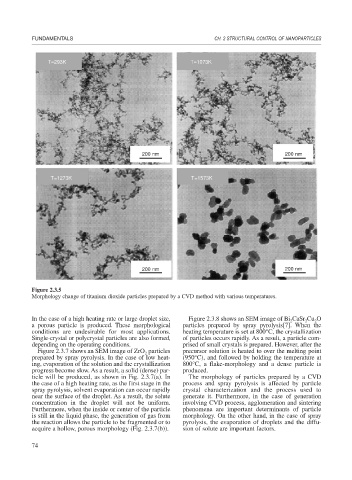
Figure 2.3.5
Morphology change of titanium dioxide particles prepared by a CVD method with various temperatures.
In the case of a high heating rate or large droplet size, Figure 2.3.8 shows an SEM image of Bi CaSr Cu O
2
2
2
a porous particle is produced. These morphological particles prepared by spray pyrolysis[7]. When the
conditions are undesirable for most applications. heating temperature is set at 800°C, the crystallization
Single-crystal or polycrystal particles are also formed, of particles occurs rapidly. As a result, a particle com-
depending on the operating conditions. prised of small crystals is prepared. However, after the
Figure 2.3.7 shows an SEM image of ZrO particles precursor solution is heated to over the melting point
2
prepared by spray pyrolysis. In the case of low heat- (950°C), and followed by holding the temperature at
ing, evaporation of the solution and the crystallization 800°C, a flake-morphology and a dense particle is
progress become slow. As a result, a solid (dense) par- produced.
ticle will be produced, as shown in Fig. 2.3.7(a). In The morphology of particles prepared by a CVD
the case of a high heating rate, as the first stage in the process and spray pyrolysis is affected by particle
spray pyrolysis, solvent evaporation can occur rapidly crystal characterization and the process used to
near the surface of the droplet. As a result, the solute generate it. Furthermore, in the case of generation
concentration in the droplet will not be uniform. involving CVD process, agglomeration and sintering
Furthermore, when the inside or center of the particle phenomena are important determinants of particle
is still in the liquid phase, the generation of gas from morphology. On the other hand, in the case of spray
the reaction allows the particle to be fragmented or to pyrolysis, the evaporation of droplets and the diffu-
acquire a hollow, porous morphology (Fig. 2.3.7(b)). sion of solute are important factors.
74