Page 96 - Book Hosokawa Nanoparticle Technology Handbook
P. 96
FUNDAMENTALS CH. 2 STRUCTURAL CONTROL OF NANOPARTICLES
(a) (b)
20 nm
50 nm
(c)
5 nm
50 nm
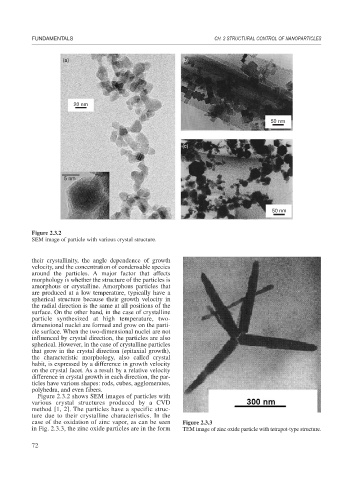
Figure 2.3.2
SEM image of particle with various crystal structure.
their crystallinity, the angle dependence of growth
velocity, and the concentration of condensable species
around the particles. A major factor that affects
morphology is whether the structure of the particles is
amorphous or crystalline. Amorphous particles that
are produced at a low temperature, typically have a
spherical structure because their growth velocity in
the radial direction is the same at all positions of the
surface. On the other hand, in the case of crystalline
particle synthesized at high temperature, two-
dimensional nuclei are formed and grow on the parti-
cle surface. When the two-dimensional nuclei are not
influenced by crystal direction, the particles are also
spherical. However, in the case of crystalline particles
that grow in the crystal direction (epitaxial growth),
the characteristic morphology, also called crystal
habit, is expressed by a difference in growth velocity
on the crystal facet. As a result by a relative velocity
difference in crystal growth in each direction, the par-
ticles have various shapes: rods, cubes, agglomerates,
polyhedra, and even fibers.
Figure 2.3.2 shows SEM images of particles with
various crystal structures produced by a CVD
method [1, 2]. The particles have a specific struc-
ture due to their crystalline characteristics. In the
case of the oxidation of zinc vapor, as can be seen Figure 2.3.3
in Fig. 2.3.3, the zinc oxide particles are in the form TEM image of zinc oxide particle with tetrapot-type structure.
72