Page 92 - Book Hosokawa Nanoparticle Technology Handbook
P. 92
FUNDAMENTALS CH. 2 STRUCTURAL CONTROL OF NANOPARTICLES
than that of the latter one. The reason why such fine We have introduced several examples of metal,
and well-dispersed particles can be synthesized from metal oxide, and sulfide nanoparticles, each synthe-
the mixture of (La(OH) Co(OH) NaCl) by the sized by MC solid-state reaction based on dry grind-
2
3
MC method is attributed to the presence of NaCl in ing starting mixtures.
the heating, and NaCl plays a big role to prevent from As is known, grinding and mechanochemistry are
agglomeration and sintering during heating, while the traditional operation and subject, but they have
aggregated particles can be obtained by the MC intrinsic properties including novel technology for
method of (La O Co O ). synthesizing nanoparticles. This technology has a lot
2
3
2
3
of potential to be able to synthesize nanoparticles of
d) Metal sulfide nanoparticles [11, 15] different kinds of materials by the combination of
The MC method is able to synthesize metal sulfide starting mixture and subsequent operations such as
nanoparticles such as ZnS, CdS. For example the syn- leaching and heating. It is known that, in fact, the
thesis of ZnS, the reaction equations are given as energy efficiency of grinding is relatively low, how-
below. ever, it is not excessively low for the MC operation
when we compare its total costs with that using other
CaS ZnCl CaCl ZnS 2CaCl (2.2.22) operations such as high temperature and solution
2 2 2
devices. This technology of grinding operation com-
Na S CdCl 15.6NaCl CdS 17.6NaCl (2.2.23) bined with subsequent operations will be developed
2 2
further in the near future, contributing to a new mate-
rial processing. This is a great expectation of this
Subsequently, followings are the important issues to kind of research work, leading to futuristic creative
reduce the particle size of the final product: and profitable advanced material science and tech-
nologies.
• Use fine powder as starting samples.
• Use small size of media in ball milling.
• Decrease the volume ratio of metal sulfide such
References
as ZnS.
[1] P.G. McCormick: Mater. Trans. JIM, 36, 161 (1995).
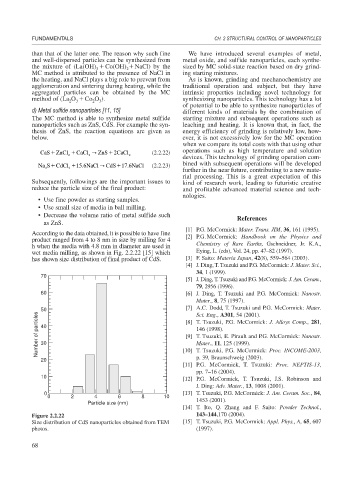
According to the data obtained, it is possible to have fine [2] P.G. McCormick: Handbook on the Physics and
product ranged from 4 to 8 nm in size by milling for 4
h when the media with 4.8 mm in diameter are used in Chemistry of Rare Earths, Gschneidner, Jr. K.A.,
wet media milling, as shown in Fig. 2.2.22 [15] which Eying, L. (eds), Vol. 24, pp. 47–82 (1997).
has shown size distribution of final product of CdS. [3] F. Saito: Materia Japan, 42(8), 559–564 (2003).
[4] J. Ding, T. Tsuzuki and P.G. McCormick: J. Mater. Sci.,
34, 1 (1999).
70
[5] J. Ding, T. Tsuzuki and P.G. McCormick: J. Am. Ceram.,
79, 2956 (1996).
60 [6] J. Ding, T. Tsuzuki and P.G. McCormick: Nanostr.
Mater., 8, 75 (1997).
50 [7] A.C. Dodd, T. Tsuzuki and P.G. McCormick: Mater.
Number of particles 40 [8] T. Tsuzuki, P.G. McCormick: J. Alloys Comp., 281,
Sci. Eng., A301, 54 (2001).
146 (1998).
[9] T. Tsuzuki, E. Pirault and P.G. McCormick: Nanostr.
30
Mater., 11, 125 (1999).
p. 39, Braunschweig (2003).
20 [10] T. Tsuzuki, P.G. McCormick: Proc. INCOME-2003,
[11] P.G. McCormick, T. Tsuzuki: Proc. NEPTIS-13,
pp. 7–16 (2004).
10
[12] P.G. McCormick, T. Tsuzuki, J.S. Robinson and
J. Ding: Adv. Mater., 13, 1008 (2001).
0 [13] T. Tsuzuki, P.G. McCormick: J. Am. Ceram. Soc., 84,
0 2 4 6 8 10
Particle size (nm) 1453 (2001).
[14] T. Ito, Q. Zhang and F. Saito: Powder Technol.,
Figure 2.2.22 143–144,170 (2004).
Size distribution of CdS nanoparticles obtained from TEM [15] T. Tsuzuki, P.G. McCormick: Appl. Phys., A, 65, 607
photos. (1997).
68