Page 88 - Book Hosokawa Nanoparticle Technology Handbook
P. 88
FUNDAMENTALS CH. 2 STRUCTURAL CONTROL OF NANOPARTICLES
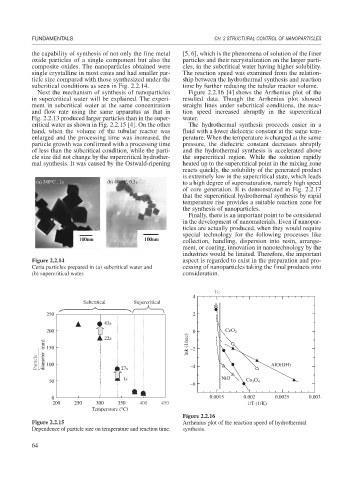
the capability of synthesis of not only the fine metal [5, 6], which is the phenomena of solution of the finer
oxide particles of a single component but also the particles and their recrystalization on the larger parti-
composite oxides. The nanoparticles obtained were cles, in the subcritical water having higher solubility.
single crystalline in most cases and had smaller par- The reaction speed was examined from the relation-
ticle size compared with those synthesized under the ship between the hydrothermal synthesis and reaction
subcritical conditions as seen in Fig. 2.2.14. time by further reducing the tubular reactor volume.
Next the mechanism of synthesis of nanoparticles Figure 2.2.16 [4] shows the Arrhenius plot of the
in supercritical water will be explained. The experi- resulted data. Though the Arrhenius plot showed
ment in subcritical water at the same concentration straight lines under subcritical conditions, the reac-
and flow rate using the same apparatus as that in tion speed increased abruptly in the supercritical
Fig. 2.2.13 produced larger particles than in the super- water.
critical water as shown in Fig. 2.2.15 [4]. On the other The hydrothermal synthesis proceeds easier in a
hand, when the volume of the tubular reactor was fluid with a lower dielectric constant at the same tem-
enlarged and the processing time was increased, the perature. When the temperature is changed at the same
particle growth was confirmed with a processing time pressure, the dielectric constant decreases abruptly
of less than the subcritical condition, while the parti- and the hydrothermal synthesis is accelerated above
cle size did not change by the supercritical hydrother- the supercritical region. While the solution rapidly
mal synthesis. It was caused by the Ostwald-ripening heated up to the supercritical point in the mixing zone
reacts quickly, the solubility of the generated product
is extremely low in the supercritical state, which leads
(a) 340°C , 1s (b) 400°C, 0.3s to a high degree of supersaturation, namely high speed
of core generation. It is demonstrated in Fig. 2.2.17
that the supercritical hydrothermal synthesis by rapid
temperature rise provides a suitable reaction zone for
the synthesis of nanoparticles.
Finally, there is an important point to be considered
in the development of nanomaterials. Even if nanopar-
ticles are actually produced, when they would require
special technology for the following processes like
100nm
100nm 100nm collection, handling, dispersion into resin, arrange-
100nm
ment, or coating, innovation in nanotechnology by the
industries would be limited. Therefore, the important
Figure 2.2.14 aspect is regarded to exist in the preparation and pro-
Ceria particles prepared in (a) subcritical water and cessing of nanoparticles taking the final products into
(b) supercritical water. consideration.
Tc
4
Subcritical Supercritical
250 2
43s
200 0 CeO 2
22s lnk (1/sec) −2
150
Particle diameter (nm) 100 27s −4 AlO(OH)
1s NiO
O
50 Co 3 4
−6
0 0.0015 0.002 0.0025 0.003
200 250 300 350 400 450 1/T (1/K)
Temperature (°C)
Figure 2.2.16
Figure 2.2.15 Arrhenius plot of the reaction speed of hydrothermal
Dependence of particle size on temperature and reaction time. synthesis.
64