Page 89 - Book Hosokawa Nanoparticle Technology Handbook
P. 89
2.2 PARTICLE SIZE FUNDAMENTALS
Intermediate to be disaggregated by a mechanical method such as
an agitation mill with small beads.
As McCormick et al. [1, 2] have already reported, a
Crystal
mechanochemical (MC) method gives us an alterna-
Subcritical tive for producing nanoparticles, and there has been a
(573K) growing interest in these days. The nanoparticles pro-
duced by this method have unique characteristics such
as narrow size distribution of particles besides well-
CeO 2 dispersible powder. Of course, it is very important to
3+
Ce ion Particles choose the suitable condition in the milling operation.
Crystal
The MC method can produce nanoparticles of metals,
metal oxides, complex oxides, and others.
Synthesis of nanoparticles of different materials by
Supercritical
(673K) solid-phase method has been introduced as below:
2.2.4.1 Mechanochemistry and solid-state reaction
When different kinds of solids are subjected to grind-
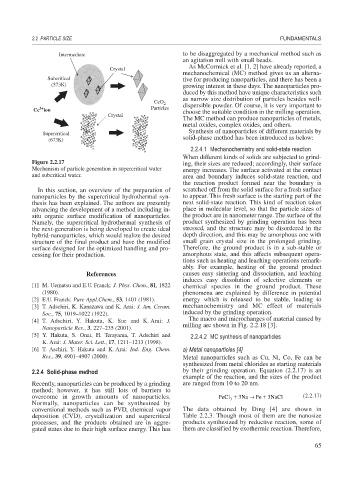
Figure 2.2.17 ing, their sizes are reduced; accordingly, their surface
Mechanism of particle generation in supercritical water energy increases. The surface activated at the contact
and subcritical water. area and boundary induces solid-state reaction, and
the reaction product formed near the boundary is
In this section, an overview of the preparation of scratched off from the solid surface for a fresh surface
nanoparticles by the supercritical hydrothermal syn- to appear. This fresh surface is the starting part of the
thesis has been explained. The authors are presently next solid-state reaction. This kind of reaction takes
advancing the development of a method including in- place in molecular level, so that the particle sizes of
situ organic surface modification of nanoparticles. the product are in nanometer range. The surface of the
Namely, the supercritical hydrothermal synthesis of product synthesized by grinding operation has been
the next-generation is being developed to create ideal stressed, and the structure may be disordered in the
hybrid-nanoparticles, which would realize the desired depth direction, and this may be amorphous one with
structure of the final product and have the modified small grain crystal size in the prolonged grinding.
surface designed for the optimized handling and pro- Therefore, the ground product is in a sub-stable or
cessing for their production. amorphous state, and this affects subsequent opera-
tions such as heating and leaching operations remark-
ably. For example, heating of the ground product
References causes easy sintering and dissociation, and leaching
induces easy dissolution of selective elements or
[1] M. Uematsu and E.U. Franck: J. Phys. Chem., 81, 1822 chemical species in the ground product. These
(1980). phenomena are explained by difference in potential
[2] E.U. Franck: Pure Appl.Chem., 53, 1401 (1981). energy which is released to be stable, leading to
[3] T. Adschiri, K. Kanazawa and K. Arai: J. Am. Ceram. mechanochemistry and MC effect of materials
Soc., 75, 1019–1022 (1922). induced by the grinding operation.
The macro and microchanges of material caused by
[4] T. Adschiri, Y. Hakuta, K. Sue and K. Arai: J.
milling are shown in Fig. 2.2.18 [3].
Nanoparticle Res., 3, 227–235 (2001).
[5] Y. Hakuta, S. Onai, H. Terayama, T. Adschiri and 2.2.4.2 MC synthesis of nanoparticles
K. Arai: J. Mater. Sci. Lett., 17, 1211–1213 (1998).
[6] T. Aschiri, Y. Hakuta and K. Arai: Ind. Eng. Chem. a) Metal nanoparticles [4]
Res., 39, 4901–4907 (2000). Metal nanoparticles such as Cu, Ni, Co, Fe can be
synthesized from metal chlorides as starting materials
2.2.4 Solid-phase method by their grinding operation. Equation (2.2.17) is an
example of the reaction, and the sizes of the product
Recently, nanoparticles can be produced by a grinding are ranged from 10 to 20 nm.
method; however, it has still lots of barriers to
overcome in growth amounts of nanoparticles. FeCl 3Na Fe 3NaCl (2.2.17)
3
Normally, nanoparticles can be synthesized by
conventional methods such as PVD, chemical vapor The data obtained by Ding [4] are shown in
deposition (CVD), crystallization and supercritical Table 2.2.3. Though most of them are the nanosize
processes, and the products obtained are in aggre- products synthesized by reductive reaction, some of
gated states due to their high surface energy. This has them are classified by exothermic reaction. Therefore,
65