Page 85 - Book Hosokawa Nanoparticle Technology Handbook
P. 85
2.2 PARTICLE SIZE FUNDAMENTALS
extreme case in which homogeneous nucleation does the model predictions are in good agreement with the
not occur at all, so that the total number concentration results obtained in liquid-phase nucleation experiments.
of particles in the system is simply equal to the num- When the seed number concentrationn is very low, the
p
ber concentration of seeds. As seen in these figures, total number concentration of particles in the system is
practically constant, independent of n . The reason for
p
this is that when n is very low, the generation rate of
p
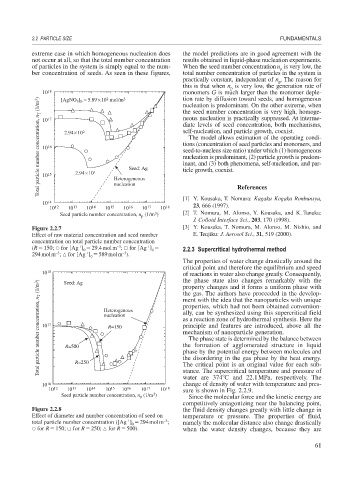
10 18 [AgNO ] = 5.89 ×10 mol/m 3 monomers G is much larger than the monomer deple-
tion rate by diffusion toward seeds, and homogeneous
Total particle number concentration, n T (1/m 3 ) 10 16 2.94×10 2 1 Seed: Ag diate levels of seed concentration, both mechanisms,
2
3 0
nucleation is predominant. On the other extreme, when
the seed number concentration is very high, homoge-
neous nucleation is practically suppressed. At interme-
17
self-nucleation, and particle growth, coexist.
The model allows estimation of the operating condi-
tions (concentration of seed particles and monomers, and
10
seed-to-nucleus size ratio) under which (1) homogeneous
nucleation is predominant, (2) particle growth is predom-
inant, and (3) both phenomena, self-nucleation, and par-
ticle growth, coexist.
2.94×10
10
15
Heterogeneous
nucleation
References
[1] Y. Kousaka, T. Nomura: Kagaku Kogaku Ronbunsyu,
10 14
10 12 10 13 10 14 10 15 10 16 10 17 10 18 23, 666 (1997).
3
Seed particle number concentration, n (1/m ) [2] T. Nomura, M. Alonso, Y. Kousaka, and K. Tanaka:
p
J. Colloid Interface Sci., 203, 170 (1998).
Figure 2.2.7 [3] Y. Kousaka, T. Nomura, M. Alonso, M. Nishio, and
Effect of raw material concentration and seed number E. Tenjiku: J. Aerosol Sci., 31, 519 (2000).
concentration on total particle number concentration
–3
(R 150; for [Ag ] 29.4molm ; for [Ag ] 2.2.3 Supercritical hydrothermal method
0
0
–3
–3
294molm ; for [Ag ] 589molm ).
0
The properties of water change drastically around the
critical point and therefore the equilibrium and speed
10 18 Seed: Ag of reactions in water also change greatly. Consequently,
the phase state also changes remarkably with the
Total particle number concentration, n T (1/m 3 ) 10 17 R=500 Heterogenous ment with the idea that the nanoparticles with unique
property changes and it forms a uniform phase with
the gas. The authors have proceeded in the develop-
properties, which had not been obtained convention-
ally, can be synthesized using this supercritical field
nucleation
as a reaction zone of hydrothermal synthesis. Here the
principle and features are introduced, above all the
R=150
mechanism of nanoparticle generation.
The phase state is determined by the balance between
the formation of agglomerated structure in liquid
phase by the potential energy between molecules and
the disordering in the gas phase by the heat energy.
R=250
The critical point is an original value for each sub-
stance. The supercritical temperature and pressure of
water are 374 C and 22.1MPa, respectively. The
10 16 change of density of water with temperature and pres-
10 12 10 13 10 14 10 15 10 16 10 17 10 18 sure is shown in Fig. 2.2.9.
3
Seed particle number concentration, n (1/m ) Since the molecular force and the kinetic energy are
p
competitively antagonizing near the balancing point,
Figure 2.2.8 the fluid density changes greatly with little change in
Effect of diameter and number concentration of seed on temperature or pressure. The properties of fluid,
total particle number concentration ([Ag ] 294molm ; namely the molecular distance also change drastically
–3
0
for R 150; for R 250; for R 500). when the water density changes, because they are
61