Page 80 - Book Hosokawa Nanoparticle Technology Handbook
P. 80
FUNDAMENTALS CH. 2 STRUCTURAL CONTROL OF NANOPARTICLES
2.2 Particle size 20 10 16
2.2.1 Gas-phase method 10 15
Homogeneous nucleation without seeds and heteroge- 15
neous nucleation with seeds have attracted much inter- 10 14
est in the wide field of particle formation from gases
and liquids by phase changes. Although many nucle- Mean volume diameter, d v (nm) 10 10 13 Number concentration, n exp * (1/m 3 )
ation theories have been proposed, the complexity of
nucleation theory prevents its practical use in applied
fields such as particle processing. 10 12
In this section, particle formation by gas-phase and 5
liquid-phase methods is described systematically. A 11
simple model of liquid-phase nucleation and its 10
expanded gas-phase nucleation model are introduced
[1–3]. These models enable prediction of the effects 0 10 10
of the operating conditions of particle processing on 10 -11 10 -10 10 -9 10 -8 10 -7 10 -6 10 -5
the steady-state concentration and size of the nucle- Monomer concentration, C ,exp (mol/m )
3
ated particle. f
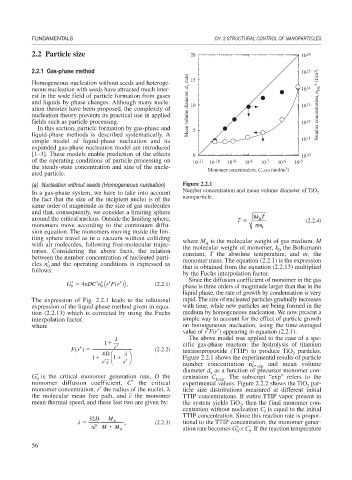
(a) Nucleation without seeds (Homogeneous nucleation) Figure 2.2.1
In a gas-phase system, we have to take into account Number concentration and mean volume diameter of TiO 2
the fact that the size of the incipient nuclei is of the nanoparticle.
same order of magnitude as the size of gas molecules
and that, consequently, we consider a limiting sphere
around the critical nucleus. Outside the limiting sphere, c 8 kT (2.2.4)
B
monomers move according to the continuum diffu- m 1
sion equation. The monomers moving inside the lim-
iting sphere travel as in a vacuum without colliding where M is the molecular weight of gas medium, M
with air molecules, following free-molecular trajec- the molecular weight of monomer, k the Boltzmann
A
B
tories. Considering the above facts, the relation constant, T the absolute temperature, and m the
between the number concentration of nucleated parti- monomer mass. The equation (2.2.1) is the expression
1
*
cles n and the operating conditions is expressed as that is obtained from the equation (2.2.13) multiplied
0
follows:
by the Fuchs interpolation factor.
Since the diffusion coefficient of monomer in the gas
*
G 4 DC n r F r() , (2.2.1) phase is three orders of magnitude larger than that in the
*
* *
*
0
0
liquid phase, the rate of growth by condensation is very
The expression of Fig. 2.2.1 leads to the relational rapid. The size of nucleated particles gradually increases
expression of the liquid-phase method given in equa- with time, while new particles are being formed in the
tion (2.2.13) which is corrected by using the Fuchs medium by homogeneous nucleation. We now present a
interpolation factor. simple way to account for the effect of particle growth
where on homogeneous nucleation, using the time-averaged
*
*
value of r F(r ) appearing in equation (2.2.1).
The above model was applied to the case of a spe-
1 cific gas-phase reaction: the hydrolysis of titanium
Fr () r * (2.2.2) tetraisopropoxide (TTIP) to produce TiO particles.
*
4 D ⎛ ⎞ 2
1 * ⎜ 1 * ⎟ Figure 2.2.1 shows the experimental results of particle
rc ⎝ r ⎠ number concentration n , and mean volume
*
0 exp
diameter d as a function of precursor monomer con-
v
*
G is the critical monomer generation rate, D the centration C f,exp . The subscript “exp” refers to the
0
monomer diffusion coefficient, C * the critical experimental values. Figure 2.2.2 shows the TiO par-
2
monomer concentration, r the radius of the nuclei, ticle size distributions measured at different initial
*
the molecular mean free path, and c the monomer TTIP concentrations. If entire TTIP vapor present in
mean thermal speed, and these last two are given by: the system yields TiO , then the final monomer con-
2
centration without nucleation C is equal to the initial
f
TTIP concentration. Since this reaction rate is propor-
32D M
A , (2.2.3) tional to the TTIP concentration, the monomer gener-
c M M A ation rate becomes G C . If the reaction temperature
*
f
0
56