Page 81 - Book Hosokawa Nanoparticle Technology Handbook
P. 81
2.2 PARTICLE SIZE FUNDAMENTALS
10 14
3
C TTIP (mol/m )
Particle number concentration, dn exp * /dlnd p (1/m 3 ) 10 13 5.94 × 10 -5
-5
3.30 × 10
-5
4.24 × 10
-5
4.95 × 10
-4
1.51 × 10
-4
1.94 × 10
-4
2.38 × 10
12
10
10 11
1 10 100
Particle diameter, d (nm)
p
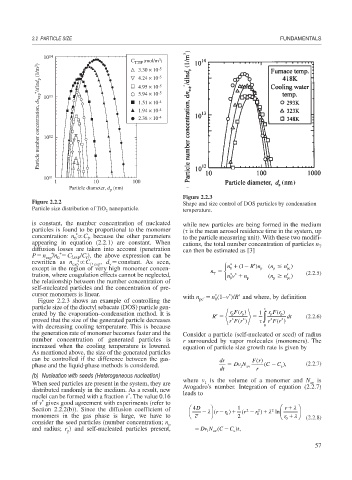
Figure 2.2.3
Figure 2.2.2 Shape and size control of DOS particles by condensation
Particle size distribution of TiO nanoparticle. temperature.
2
is constant, the number concentration of nucleated while new particles are being formed in the medium
particles is found to be proportional to the monomer ( is the mean aerosol residence time in the system, up
*
concentration: n C , because the other parameters to the particle measuring unit). With these two modifi-
0
f
appearing in equation (2.2.1) are constant. When cations, the total number concentration of particles n T
diffusion losses are taken into account (penetration can then be estimated as [3]
*
P n exp * /n C f,exp /C ), the above expression can be
f
0
rewritten as n exp * C , , d constant. As seen, ⎧ * R n ) ′ n ′
f exp
v
except in the region of very high monomer concen- n ⎨ ⎪ n 1( p ( p n )
0
pc
tration, where coagulation effects cannot be neglected, T ⎩ ⎪ nv n p ( n ′ pc (2.2.5)
**
n )
0
p
the relationship between the number concentration of
self-nucleated particles and the concentration of pre-
cursor monomers is linear. with n n (1– )/R and where, by definition
*
*
Figure 2.2.3 shows an example of controlling the pc 0
particle size of the dioctyl sebacate (DOS) particle gen-
erated by the evaporation–condensation method. It is ′ R rF r () 1 ∫ rF r () dt (2.2.6)
p
p
p
p
proved that the size of the generated particle decreases rF r () rF r ()
*
*
*
*
with decreasing cooling temperature. This is because 0
the generation rate of monomer becomes faster and the Consider a particle (self-nucleated or seed) of radius
number concentration of generated particles is r surrounded by vapor molecules (monomers). The
increased when the cooling temperature is lowered. equation of particle size growth rate is given by
As mentioned above, the size of the generated particles
can be controlled if the difference between the gas- dr Fr ()
phase and the liquid-phase methods is considered. Dv N av ( C C ), (2.2.7)
1
s
dt r
(b) Nucleation with seeds (Heterogeneous nucleation)
where is the volume of a monomer and N is
av
When seed particles are present in the system, they are Avogadro’s number. Integration of equation (2.2.7)
1
distributed randomly in the medium. As a result, new leads to
*
nuclei can be formed with a fraction . The value 0.16
*
of gives good agreement with experiments (refer to ⎛
⎞
Section 2.2.2(b)). Since the diffusion coefficient of ⎛ ⎜ 4D ( r ) 1 r r ln ⎜ r ⎞ ⎟
2
)
2
⎟
r (
2
monomers in the gas phase is large, we have to ⎝ c ⎠ 0 2 0 ⎝ r ⎠ (2.2.8)
0
consider the seed particles (number concentration; n p
and radius; r ) and self-nucleated particles present, Dv N ( C C )),t
p 1 av s
57