Page 97 - Book Hosokawa Nanoparticle Technology Handbook
P. 97
2.3 PARTICLE SHAPE FUNDAMENTALS
of a tetrapot-type structure, also called four-rings, the reactor, is longer than (A region). In this case,
f
c
consisting of four needles [3]. It is generally the particles grow by coagulation, and are spherical
thought that this structure is due to the larger because the sintering ends instantaneously. Therefore,
growth velocity in the C axis than in the other axes. the particle diameter depends on the frequency of the
Therefore, when the zinc vapor content decreases, collisions between the particles greatly. On the other
the particles do not grow to a needle shape and hand, when is longer than (B region), as mentioned
c
f
become an aggregate consisting of ultrafine spheri- above, the particles are agglomerated. We can con-
cal particles. clude that the morphology of agglomerated particles
Depending on coagulation and sintering behavior, is dependent on both the coagulation and sintering
the morphology of particles synthesized by a CVD behavior.
method can be spherical or agglomerates [4]. When Figure 2.3.5 shows the change in morphology of
the characteristic time for coagulation ( ), i.e. the titanium dioxide particles synthesized by a CVD
c
velocity of coagulation, is sufficiently shorter than the method at various temperatures [5]. The titanium
characteristic time for sintering ( ), i.e. the velocity dioxide particles were synthesized by the thermal
f
of sintering, the particles first become nonspherical decomposition of tetraisopropoxide (TTIP). In all
agglomerates. These nonspherical aggregates gradually cases, the particles are agglomerations of the
become spherical due to the sintering after . nanometer order of primary particles, formed at a
f
However, when another particle agglutinates before f low reaction temperature. When the reaction
passes, the particles are agglomerated. That is to say, temperature increases above 800 C, where titanium
at , the particles are spherical, at , the par- dioxide particles undergo sintering, the primary
c
f
f
c
ticles are nonspherical aggregates. The equation for particles are larger because they are growing up by
expressing has the functions of sintering mecha- sintering.
f
nism, particle properties (melting point, diffusion coef- In general, nanoparticles generated by CVD
ficient, and so on), temperature, and size. Consequently, processes are produced in the form of aggregates
it is important to evaluate coagulation and the sinter- due to coagulation. An electrospray-assisted CVD
ing phenomena in the characterization of the size of (ES-CVD) method has recently been reported for
product particles. Both phenomena have an effect on generating nonagglomerated nanoparticles because
the temperature profile during the particle production unipolarly charged particles undergo mutual electro-
process. static repulsions, and particle collisions and growth
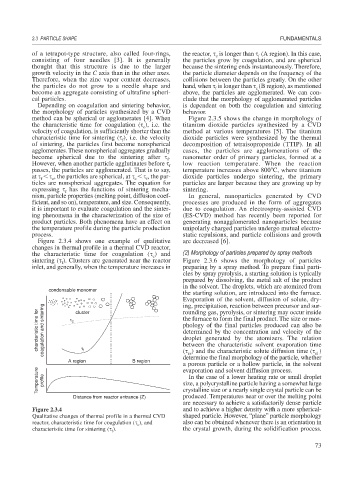
Figure 2.3.4 shows one example of qualitative are decreased [6].
changes in thermal profile in a thermal CVD reactor,
the characteristic time for coagulation ( ) and (2) Morphology of particles prepared by spray methods
c
sintering ( ). Clusters are generated near the reactor Figure 2.3.6 shows the morphology of particles
f
inlet, and generally, when the temperature increases in preparing by a spray method. To prepare final parti-
cles by spray pyrolysis, a starting solution is typically
prepared by dissolving, the metal salt of the product
in the solvent. The droplets, which are atomized from
condensable monomer
the starting solution, are introduced into the furnace.
Evaporation of the solvent, diffusion of solute, dry-
ing, precipitation, reaction between precursor and sur-
charcteristic time for coagulation and sintering τ c the furnace to form the final product. The size or mor-
rounding gas, pyrolysis, or sintering may occur inside
cluster
phology of the final particles produced can also be
determined by the concentration and velocity of the
droplet generated by the atomizers. The relation
between the characteristic solvent evaporation time
τ f
sl
sv
determine the final morphology of the particle, whether
A region B region ( ) and the characteristic solute diffusion time ( )
a porous particle or a hollow particle, in the solvent
Temperature profile size, a polycrystalline particle having a somewhat large
evaporation and solvent diffusion process.
In the case of a lower heating rate or small droplet
produced. Temperatures near or over the melting point
Distance from reactor entrance (Z) crystalline size or a nearly single crystal particle can be
are necessary to achieve a satisfactorily dense particle
Figure 2.3.4 and to achieve a higher density with a more spherical-
Qualitative changes of thermal profile in a thermal CVD shaped particle. However, “plane” particle morphology
reactor, characteristic time for coagulation ( ), and also can be obtained whenever there is an orientation in
c
characteristic time for sintering ( ). the crystal growth, during the solidification process.
f
73