Page 221 - Book Hosokawa Nanoparticle Technology Handbook
P. 221
4.3 NANOPORE STRUCTURE FUNDAMENTALS
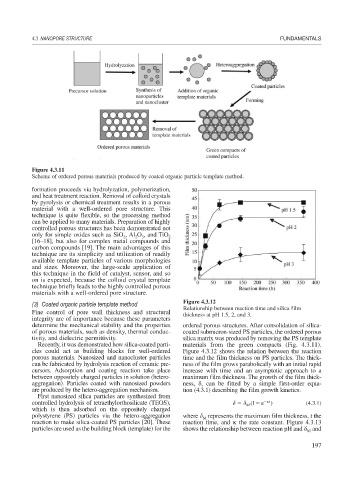
Figure 4.3.11
Scheme of ordered porous materials produced by coated organic particle template method.
formation proceeds via hydrolyzation, polymerization,
and heat treatment reaction. Removal of colloid crystals
by pyrolysis or chemical treatment results in a porous
material with a well-ordered pore structure. This
technique is quite flexible, so the processing method
can be applied to many materials. Preparation of highly
controlled porous structures has been demonstrated not
only for simple oxides such as SiO , Al O , and TiO 2
2
2
3
[16–18], but also for complex metal compounds and
carbon compounds [19]. The main advantages of this
technique are its simplicity and utilization of readily
available template particles of various morphologies
and sizes. Moreover, the large-scale application of
this technique in the field of catalyst, sensor, and so
on is expected, because the colloid crystal template
technique briefly leads to the highly controlled porous
materials with a well-ordered pore structure.
(3) Coated organic particle template method Figure 4.3.12
Relationship between reaction time and silica film
Fine control of pore wall thickness and structural thickness at pH 1.5, 2, and 3.
integrity are of importance because these parameters
determine the mechanical stability and the properties ordered porous structures. After consolidation of silica-
of porous materials, such as density, thermal conduc- coated submicron-sized PS particles, the ordered porous
tivity, and dielectric permittivity. silica matrix was produced by removing the PS template
Recently, it was demonstrated how silica-coated parti- materials from the green compacts (Fig. 4.3.11).
cles could act as building blocks for well-ordered Figure 4.3.12 shows the relation between the reaction
porous materials. Nanosized and nanocluster particles time and the film thickness on PS particles. The thick-
can be fabricated by hydrolysis reaction of ceramic pre- ness of the film grows parabolically with an initial rapid
cursors. Adsorption and coating reaction take place increase with time and an asymptotic approach to a
between oppositely charged particles in solution (hetero- maximum film thickness. The growth of the film thick-
aggregation). Particles coated with nanosized powders ness, , can be fitted by a simple first-order equa-
are produced by the hetero-aggregation mechanism. tion (4.3.1) describing the film growth kinetics.
First nanosized silica particles are synthesized from
controlled hydrolysis of tetraethylorthosilicate (TEOS), (1 e t ) (4.3.1)
M
which is then adsorbed on the oppositely charged
polystyrene (PS) particles via the hetero-aggregation where represents the maximum film thickness, t the
M
reaction to make silica-coated PS particles [20]. These reaction time, and the rate constant. Figure 4.3.13
particles are used as the building block (template) for the shows the relationship between reaction pH and and
M
197