Page 220 - Book Hosokawa Nanoparticle Technology Handbook
P. 220
FUNDAMENTALS CH. 4 CONTROL OF NANOSTRUCTURE OF MATERIALS
4.3.3 Ordered porous structures (2) Colloid crystal template method
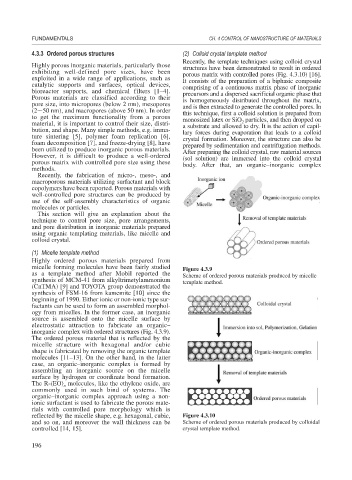
Recently, the template techniques using colloid crystal
Highly porous inorganic materials, particularly those structures have been demonstrated to result in ordered
exhibiting well-defined pore sizes, have been porous matrix with controlled pores (Fig. 4.3.10) [16].
exploited in a wide range of applications, such as It consists of the preparation of a biphasic composite
catalytic supports and surfaces, optical devices, comprising of a continuous matrix phase of inorganic
bioreactor supports, and chemical filters [1–4]. precursors and a dispersed sacrificial organic phase that
Porous materials are classified according to their is homogeneously distributed throughout the matrix,
pore size, into micropores (below 2 nm), mesopores and is then extracted to generate the controlled pores. In
(2 50 nm), and macropores (above 50 nm). In order this technique, first a colloid solution is prepared from
to get the maximum functionality from a porous monosized latex or SiO particles, and then dropped on
2
material, it is important to control their size, distri- a substrate and allowed to dry. It is the action of capil-
bution, and shape. Many simple methods, e.g. imma- lary forces during evaporation that leads to a colloid
ture sintering [5], polymer foam replication [6], crystal formation. Moreover, the structure can also be
foam decomposition [7], and freeze-drying [8], have prepared by sedimentation and centrifugation methods.
been utilized to produce inorganic porous materials. After preparing the colloid crystal, raw material sources
However, it is difficult to produce a well-ordered (sol solution) are immersed into the colloid crystal
porous matrix with controlled pore size using these body. After that, an organic–inorganic complex
methods.
Recently, the fabrication of micro-, meso-, and
macroporous materials utilizing surfactant and block
copolymers have been reported. Porous materials with
well-controlled pore structures can be produced by
use of the self-assembly characteristics of organic
molecules or particles.
This section will give an explanation about the
technique to control pore size, pore arrangements,
and pore distribution in inorganic materials prepared
using organic templating materials, like micelle and
colloid crystal.
(1) Micelle template method
Highly ordered porous materials prepared from
micelle forming molecules have been fairly studied Figure 4.3.9
as a template method after Mobil reported the Scheme of ordered porous materials produced by micelle
synthesis of MCM-41 from alkyltrimetylammonium template method.
(CnTMA) [9] and TOYOTA group demonstrated the
synthesis of FSM-16 from kamemite [10] since the
beginning of 1990. Either ionic or non-ionic type sur-
factants can be used to form an assembled morphol-
ogy from micelles. In the former case, an inorganic
source is assembled onto the micelle surface by
electrostatic attraction to fabricate an organic–
inorganic complex with ordered structures (Fig. 4.3.9).
The ordered porous material that is reflected by the
micelle structure with hexagonal and/or cubic
shape is fabricated by removing the organic template
molecules [11–13]. On the other hand, in the latter
case, an organic–inorganic complex is formed by
assembling an inorganic source on the micelle
surface by hydrogen or coordinate bond formation.
The R-(EO) molecules, like the ethylene oxide, are
n
commonly used in such bind of systems. The
organic–inorganic complex approach using a non-
ionic surfactant is used to fabricate the porous mate-
rials with controlled pore morphology which is
reflected by the micelle shape, e.g. hexagonal, cubic, Figure 4.3.10
and so on, and moreover the wall thickness can be Scheme of ordered porous materials produced by colloidal
controlled [14, 15]. crystal template method.
196