Page 225 - Book Hosokawa Nanoparticle Technology Handbook
P. 225
4.3 NANOPORE STRUCTURE FUNDAMENTALS
Table 4.3.3
Titania nanotubes prepared by soft-chemical methods.
Author Raw material Condition of alkali treatment Product
Kasuga [14, 15] A,R
A R 5–17 M NaOH, 25–150 C TiO 2
Wang [16] A 10 M NaOH, 110 C, 20H TiO 2
Ma [17] A 10 M NaOH, 110–150 C, 12–72 H Layered titania oxide
H Ti 2-/4 O 4
x-4
x
Seo [18] A 5 M NaOH, 100–200 C, 12H TiO 2
Yao [19] A 10 M NaOH, 150 C, 12H TiO 2
Chen [20] A 10 M NaOH, 130 C, 72H
Du [21] A 10 M NaOH, 130 C, 24–72H H Ti O 7
3
2
Sun [22] A 10 M NaOH, 100–180 C, 48H Na H Ti O
x 2-x 3 7
Note: A, anatase; R, rutile; A R, composite of anatase and rutile.
5–15M NaOH aqueous solution at 60–120ºC for 20 h,
and subsequent HCl aqueous solution. Table 4.3.3
shows some reports on titania nanotubes prepared by
soft-chemical methods.
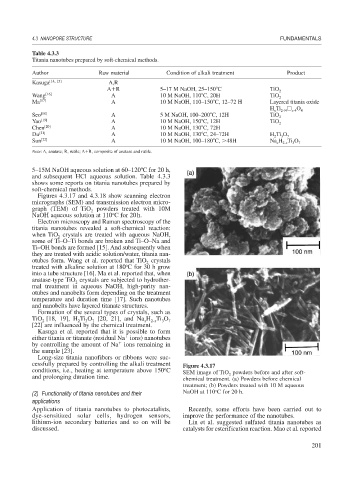
Figures 4.3.17 and 4.3.18 show scanning electron
micrographs (SEM) and transmission electron micro-
graph (TEM) of TiO powders treated with 10M
2
o
NaOH aqueous solution at 110 C for 20h.
Electron microscopy and Raman spectroscopy of the
titania nanotubes revealed a soft-chemical reaction:
when TiO crystals are treated with aqueous NaOH,
2
some of Ti–O–Ti bonds are broken and Ti–O–Na and
Ti–OH bonds are formed [15]. And subsequently when
they are treated with acidic solution/water, titania nan-
otubes form. Wang et al. reported that TiO crystals
2
o
treated with alkaline solution at 180 C for 30 h grow
into a tube structure [16]. Ma et al. reported that, when
anatase-type TiO crystals are subjected to hydrother-
2
mal treatment in aqueous NaOH, high-purity nan-
otubes and nanobelts form depending on the treatment
temperature and duration time [17]. Such nanotubes
and nanobelts have layered titanate structures.
Formation of the several types of crystals, such as
TiO [18, 19], H Ti O [20, 21], and Na H Ti O 7
2
3
7
x
2-x
2
3
[22] are influenced by the chemical treatment.
Kasuga et al. reported that it is possible to form
either titania or titanate (residual Na ions) nanotubes
by controlling the amount of Na ions remaining in
the sample [23].
Long-size titania nanofibers or ribbons were suc-
cessfully prepared by controlling the alkali treatment Figure 4.3.17
o
conditions, i.e., heating at temperature above 150 C SEM image of TiO powders before and after soft-
2
and prolonging duration time. chemical treatment. (a) Powders before chemical
treatment; (b) Powders treated with 10 M aqueous
o
(2) Functionality of titania nanotubes and their NaOH at 110 C for 20 h.
applications
Application of titania nanotubes to photocatalists, Recently, some efforts have been carried out to
dye-sensitized solar cells, hydrogen sensors, improve the performance of the nanotubes.
lithium-ion secondary batteries and so on will be Lin et al. suggested sulfated titania nanotubes as
discussed. catalysts for esterification reaction. Mao et al. reported
201