Page 227 - Book Hosokawa Nanoparticle Technology Handbook
P. 227
4.4 NANOCOMPOSITE STRUCTURE FUNDAMENTALS
[22] X. Sun, Y. Li: Chem. Eur. J., 9, 2229–2238 (2003). catalyst (support) surface
[23] T. Kasuga: Thin Solid Films, 496, 141–145 (2006).
[24] S. Kubota, K. Johkura, K. Asanuma, Y. Okouchi,
N. Ogiwara, K. Sasaki and T. Kasuga: J. Mater. Sci.
Mater., 15, 1031–1035 (2004).
4.4 Nanocomposite structure
pore pore
pore
4.4.1 Catalyst microstructure
This chapter explains the catalyst microstructure.
Though a broad range of contents are included in the
term of “catalyst microstructure”, this chapter covers
the microstructure of the solid catalyst which is
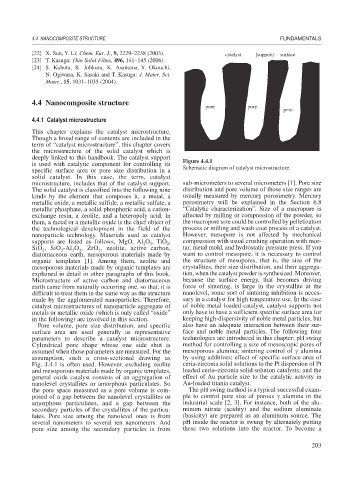
deeply linked to this handbook. The catalyst support Figure 4.4.1
is used with catalytic component for controlling its Schematic diagram of catalyst microstructure.
specific surface area or pore size distribution in a
solid catalyst. In this case, the term, catalyst
microstructure, includes that of the catalyst support. sub-micrometers to several micrometers [1]. Pore size
The solid catalyst is classified into the following nine distribution and pore volume of those size ranges are
kinds by the element that composes it, a metal, a usually measured by mercury porosimetry. Mercury
metallic oxide, a metallic sulfide, a metallic sulfate, a porosimetry will be explained in the Section 6.8
metallic phosphate, a solid phosphoric acid, a cation- “Catalytic characterization”. Size of a macropore is
exchange resin, a zeolite, and a heteropoly acid. In affected by milling or compression of the powder, so
them, a metal or a metallic oxide is the chief object of the macropore size could be controlled by pelletization
the technological development in the field of the process or milling and wash-coat process of a catalyst.
nanoparticle technology. Materials used as catalyst However, mesopore is not affected by mechanical
supports are listed as follows, MgO, Al O , TiO , compression with usual crushing operation with mor-
2
2
3
SiO , SiO -Al O , ZrO , zeolite, active carbon, tar, metal mold, and hydrostatic pressure press. If you
3
2
2
2
2
diatomaceous earth, mesoporous materials made by want to control mesopore, it is necessary to control
organic templates [1]. Among them, zeolite and the structure of mesopores, that is, the size of the
mesoporous materials made by organic templates are crystallites, their size distribution, and their aggrega-
explained in detail in other paragraphs of this book. tion, when the catalyst powder is synthesized. Moreover,
Microstructure of active carbon and diatomaceous because the surface energy, that becomes driving
earth came from naturally occurring one, so that, it is force of sintering, is large in the crystallite at the
difficult to treat them in the same way as the structure nanolevel, some sort of sintering inhibition is neces-
made by the agglomerated nanoparticles. Therefore, sary in a catalyst for high temperature use. In the case
catalyst microstructures of nanoparticle aggregate of of noble metal loaded catalyst, catalyst supports not
metals or metallic oxide (which is only called “oxide” only have to have a sufficient specific surface area for
in the following) are involved in this section. keeping high-dispersivity of noble metal particles, but
Pore volume, pore size distribution, and specific also have an adequate interaction between their sur-
surface area are used generally as representative face and noble metal particles. The following four
parameters to describe a catalyst microstructure. technologies are introduced in this chapter; pH swing
Cylindrical pore shape whose one side shut is method for controlling a size of mesoscopic pores of
assumed when those parameters are measured. For the mesoporous alumina; sintering control of alumina
assumption, such a cross-sectional drawing as by using additives; effect of specific surface area of
Fig. 4.4.1 is often used. However, excluding zeolite ceria-zirconia solid solutions to the Pt dispersion of Pt
and mesoporous materials made by organic templates, loaded ceria–zirconia solid solution catalysts; and the
general oxide catalyst consists of an aggregation of effect of Au particle size to the catalytic activity in
nanolevel crystallites or amorphous particulates. So Au-loaded titania catalyst.
the pore space measured as a pore volume is com- The pH swing method is a typical successful exam-
posed of a gap between the nanolevel crystallites or ple to control pore size of porous alumina in the
amorphous particulates, and a gap between the industrial scale [2, 3]. For instance, both of the alu-
secondary particles of the crystallites of the particu- minum nitrate (acidity) and the sodium aluminate
lates. Pore size among the nanolevel ones is from (basicity) are prepared as an aluminum source. The
several nanometers to several ten nanometers. And pH inside the reactor is swung by alternately putting
pore size among the secondary particles is from these two solutions into the reactor. To become a
203