Page 230 - Book Hosokawa Nanoparticle Technology Handbook
P. 230
FUNDAMENTALS CH. 4 CONTROL OF NANOSTRUCTURE OF MATERIALS
CO oxidation activity of Au decreases when a catalyst
support is alumina or silica. If high acidic silica-
Surface area of Pt per unit weight of each sample (m 2 /gsample) 500°C dation activity of Au/alkali earth metal catalyst tends
alumina is used as a support of Au, higher temperature
than 500 K is necessary for CO oxidation. The CO oxi-
to decrease within several months, which of Au/transi-
tion element catalyst tends to decrease within several
years. This is the reason why catalyst activity
decreases, because of an accumulation of reaction
700°C
inhibitor such as carbonate on the boundary of Au par-
ticles and the support. A lot of research reports have
900°C
been published lofty apex for Au/TiO catalyst among
2
Au-loaded catalyst. Because it is easy to obtain high-
specific surface area, easy to achieve high Au disper-
sion, and easy to refresh by heating, by the light
0 50 100 150 200 irradiation, and the moisture addition, etc. The CO
2
Specific surface area of support (m /g) oxidation rate rises by decreasing the Au particle
(ceria-zirconia solid solutions) diameter, and the catalyst activity rises by increasing
the contact boundary of the Au particle and titania.
Figure 4.4.5
Effect of specific surface area of ceria–zirconia support on
the surface area of Pt per unit weight of each sample [10]. References
[1] E. Kikuchi, K. Segawa, A. Tada, Y. Imizu and
H. Hattori: Atarashii Shokubai Kagaku, Sankyo
Syuppan (in Japanese) (1997).
[2] S. Asaoka: Shokubai, 28(4), 256–261 (1986).
[3] S. Inoue, T. Takatsuka, Y. Wada, S. Nakata and T. Ono:
Catalysis Today, 43(3–4), 225–232 (1998).
[4] H. Schaper, E.B.M. Doesburg, P.H.M. Dekorte and
L.L. Vanreijen: Solid State Ionics, 16 (1–4), 261–265
(1985).
[5] P. Burtin, J.P. Brunelle, M. Pijolat and M. Soustelle:
Appl. Catal., 34(1–2), 239–254 (1987).
[6] M.A. Fraga, E. Soares de Souza, F. Villain and
L.G. Appel: Appl. Catal. A, 259(1–8), 57–63 (2004).
[7] H. Arai, M. Machida: Shokubai, 33(5), 328–334 (1991).
[8] H. Arai, M. Machida: Shokubai, 35(4), 231–236 (1993).
[9] T. Horiuchi, L. Chen, T. Osaki, T. Sugiyama, K. Suzuki
and T. Mori1: Catal. Lett., 58(2–3), 89–92 (1999).
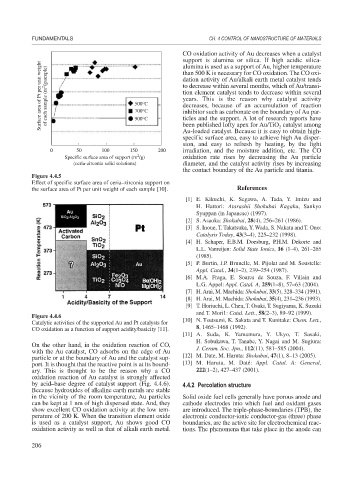
Figure 4.4.6
Catalytic activities of the supported Au and Pt catalysts for [10] N. Tsutsumi, K. Sakata and T. Kunitake: Chem. Lett.,
CO oxidation as a function of support acidity/basicity [11]. 8, 1465–1468 (1992).
[11] A. Suda, K. Yamamura, Y. Ukyo, T. Sasaki,
H. Sobukawa, T. Tanabe, Y. Nagai and M. Sugiura:
On the other hand, in the oxidation reaction of CO, J. Ceram. Soc. Jpn., 112(11), 581–585 (2004).
with the Au catalyst, CO adsorbs on the edge of Au
particle or at the boundary of Au and the catalyst sup- [12] M. Date, M. Haruta: Shokubai, 47(1), 8–13 (2005).
port. It is thought that the reactive point is at its bound- [13] M. Haruta, M. Daté: Appl. Catal. A: General,
ary. This is thought to be the reason why a CO 222(1–2), 427–437 (2001).
oxidation reaction of Au catalyst is strongly affected
by acid–base degree of catalyst support (Fig. 4.4.6). 4.4.2 Percolation structure
Because hydroxides of alkaline earth metals are stable
in the vicinity of the room temperature, Au particles Solid oxide fuel cells generally have porous anode and
can be kept at 1 nm of high dispersed state. And, they cathode electrodes into which fuel and oxidant gases
show excellent CO oxidation activity at the low tem- are introduced. The triple-phase-boundaries (TPB), the
perature of 200 K. When the transition element oxide electronic conductor-ionic conductor-gas (three) phase
is used as a catalyst support, Au shows good CO boundaries, are the active site for electrochemical reac-
oxidation activity as well as that of alkali earth metal. tions. The phenomena that take place in the anode can
206